Abstract
Recombinant (r) Mycobacterium bovis strains were constructed that secrete biologically active listeriolysin (Hly) fusion protein of Listeria monocytogenes. The r-BCG strains pAT261:Hly or pMV306:Hly expressed plasmid multicopies or chromosomal single copies of the hly gene, respectively. Human and murine macrophage-like cell lines were infected with r-BCG pAT261:Hly and pMV306:Hly strains. Interestingly, intracellular persistence of both r-BCG strains was reduced in macrophages as compared with the parental BCG strain. By immunogold labeling Hly was detected in membrane structures and within the phagosomal space of macrophages. In addition, Hly was localized within cytoplasmic vacuoles outside the mycobacteria-containing phagosome of host cells infected with r-BCG pAT261:Hly or r-BCG pMV306:Hly. Hly fusions consistently colocalized with a lysosome-associated membrane glycoprotein, suggesting that membrane-attack conformation of Hly was not altered. Although r-BCG pAT261:Hly and r-BCG pMV306:Hly microorganims apparently did not egress into the cytoplasmic compartment of host cells, they both improved major histocompatibility complex class I presentation of cophagocytosed soluble protein as compared with wild-type BCG microbes. These data suggest that Hly secretion endows BCG with an improved capacity to stimulate CD8 T cells. Because CD8 T cells play a major role in protection against tuberculosis such Hly secreting r-BCG constructs are antituberculosis vaccine candidates.
Tuberculosis (TB) caused by Mycobacterium tuberculosis remains a significant global health problem. It is estimated that one-third of the world population is infected with M. tuberculosis (1). In many countries the only measure for TB control has been vaccination with Mycobacterium bovis Bacille Calmette-Guérin (BCG). The vaccine efficacy of BCG against TB, however, shows extreme variations ranging from 0% to 80% between different field trials (2, 3). Thus, BCG should be improved, e.g., by genetic engineering to provide a vaccine for better TB control (4, 5). M. tuberculosis belongs to the group of intracellular bacteria that replicate within the phagosomal vacuoles of resting macrophages and protection against TB depends on T cell-mediated immunity (6). Most intracellular bacteria including M. tuberculosis arrest phagosome maturation and thus replicate in an altered phagosome (7). Some intracellular bacteria, such as Listeria monocytogenes egress from the phagosome and survive in the cytoplasm of host cells (8). Accordingly, it is generally assumed that antigens from pathogens remaining in the phagosome are primarily presented by major histocompatibility complex (MHC) class II molecules to CD4 T cells but bacterial antigens recognized by CD8 T cells via MHC class I gene products originate from bacteria that replicate in the cytoplasm of antigen-presenting cells. Several studies in mice and humans, however, have shown that M. tuberculosis microorganisms not only stimulate antigen-specific MHC class II-restricted CD4 T helper cells but also MHC class I-restricted CD8 cytotoxic T cells (6).
The important role of MHC class I-restricted CD8 T cells was convincingly demonstrated by the failure of β2-microglobulin (β2m)-deficient mice to control experimental M. tuberculosis infection (9). Because these mutant mice lack MHC class I, functional CD8 T cells cannot develop. In contrast to M. tuberculosis infection, β2m-deficient mice are capable of controlling lower inocula of the BCG vaccine strain (9, 10). Furthermore, BCG vaccination of β2m-deficient mice only prolonged survival after M. tuberculosis challenge infection, whereas BCG-immunized C57BL/6 mice resisted TB (9). This differential CD8 T cell dependency between M. tuberculosis and BCG may be explained as follows: M. tuberculosis antigens have better access to the host cell cytoplasm than antigens from BCG, leading to more pronounced MHC class I presentation (5). Consequently, a more effective CD8 T cell response is generated by M. tuberculosis. This notion was recently supported by in vitro experiments showing increased MHC class I presentation of a bystander antigen, soluble hen egg ovalbumin (OVA), by simultaneous M. tuberculosis, rather than BCG infection of antigen-presenting cells (11).
The phagosomal escape of L. monocytogenes represents a unique mechanism to facilitate MHC class I antigen presentation of listerial antigens (8, 12). Hly, a pore-forming sulfhydryl-activated cytolysin, is essential for the release of L. monocytogenes microorganisms from phagosomal vacuoles into the cytoplasm of host cells (12, 13). This escape function was recently transferred to Bacillus subtilis and to attenuated Salmonella spp. strains (14–16). As a corollary, r-BCG strains secreting hemolytically active Hly were constructed to exploit their efficacy in improving MHC class I-restricted immune responses and hence their applicability as candidate vaccines against TB. Herein we report on the intracellular localization of and on MHC class I antigen delivery by such r-BCG constructs.
MATERIALS AND METHODS
Bacterial Strains and Plasmids.
M. bovis BCG Chicago [American Type Culture Collection (ATCC) product 27289] was cultured in Dubos broth base (Difco) supplemented with Dubos medium albumin (Difco) at 37°C. L. monocytogenes EGD Sv 1/2a was originally obtained from G. B. Mackaness and grown as described (17). Plasmid pILH-1 was a gift from I. Gentschev and W. Goebel (University of Würzburg, Germany; ref. 15). The mycobacteria Escherichia coli shuttle vectors pAT261 and pMV306 were provided by MedImmune (Gaithersburg, MD).
Cell Lines.
The T cell hybridoma RF33.70 was provided by K. L. Rock (Harvard Medical School) (18). Interleukin (IL) 2-dependent CTLL-2 cells (ATCC product TIB-214) were maintained in human r-IL-2 at 102 units/ml (a gift from M. K. Gately, Hoffmann–La Roche). These cells and human and murine macrophage-like cells THP-1 (ATCC product TIB-202), J774A.1 (ATCC product TIB-67), and BM A3.1A7 (a gift from K. L. Rock; ref. 19) were grown under conventional cell culture conditions.
DNA Sequencing.
The correct DNA sequence of the r-plasmids, pAT261:Hly and pMV306:Hly, was determined at the sites of fragment insertion by using the following oligonucleotides BCG-Hly5 (GCTTTGTCCTGCTG) and BCG-Hly3 (GGAAGTCAGGGTGA) (Sequiserve, Vaterstetten, Germany).
Characterization of r-M. bovis BCG Strains.
The plasmids pAT261:Hly or pMV306:Hly were introduced into M. bovis BCG Chicago by standard electroporation protocol and then r-colonies were selected and grown as described (20, 21). After washing the cells in PBS plus Tween 80, the cell suspension was concentrated 20-fold in RIPA buffer (1% Nonidet P-40/0.5% deoxycholate/0.1% SDS/50 mM Tris⋅HCl, pH 8.0) to lyse the cells. Bacteria-free supernatant (1 ml) of these cultures were filtered through 0.2-μm membrane filters. The Hly fusion protein in the supernatant was enriched by incubation with 100 μl of butyl-Sepharose (Pharmacia) for 30 min at room temperature in a rotating device (M. Hess, personal communication; ref. 22). After centrifugation (Heraeus 2704 Rotor; 3,000 rpm) the pellet was dissolved in Laemmli buffer as described (17). Subsequently proteins were separated by SDS/PAGE on 10% gels and stained with the anti-Hly mAb H14–3 by chemiluminescent immunodetection as described (17, 23). The hemolytic activity of supernatants and whole-bacterial suspensions of BCG pAT261:Hly, BCG pMV306:Hly, BCG, and L. monocytogenes was determined by serially diluting the samples in PBS containing 0.1% BSA as described (15).
In Vitro Analysis of Mycobacterial Growth.
Human and murine macrophage-like cells THP-1 and J774A.1, respectively, were allowed to adhere to 24-well plates (106 cells per well). For THP-1, adherence was achieved by stimulation with 10 nM phorbol myristate acetate (Sigma) 48 h before infection. Mycobacterial growth in these cells was analyzed as described (24).
Lactate Dehydrogenase (LDH) Release.
The cytotoxicity of r-BCG strains and of L. monocytogenes EGD as positive control was determined by measuring the LDH release from infected J774A.1 macrophages. The culture supernatants and cell lysates of BCG, BCG pAT261:Hly, BCG pMV306:Hly, or L. monocytogenes EGD-infected J774A.1 macrophages were assayed for LDH activity by using the quantitation kit obtained from Promega as described (25).
Immunoelectron Microscopy Analysis.
Immunoelectron microscopy was performed on frozen sections from bone marrow-derived macrophages infected with wild-type or r-BCG 24 h before processing. Infected bone marrow-derived macrophages were fixed in 1% glutaraldehyde in Hepes saline and infiltrated in polyvinylpyrrolidone/sucrose as described (26, 27). Sections were probed with affinity-purified anti-Hly (a gift from D. Portnoy, University of Pennsylvania) and 1D4B, a rat mAb directed against lysosome-associated membrane glycoprotein (LAMP) 1. All antibodies were diluted in Hepes saline with 5% fetal calf serum and 5% goat serum. Binding of primary antibody was visualized by incubation with goat anti-rabbit IgG conjugated to 18-nm gold (Jackson Immunoresearch) and goat anti-rat IgG conjugated to 6-nm gold. Controls were conducted with wild-type BCG and by omission of the primary antibody. Under the conditions used, background labeling remained below 8% of the specific level.
Antigen Presentation Assays.
The standard assay for the presentation of exogenously added OVA antigen consisted of C57BL/6-derived BM A3.1A7 cells (stimulated with r-interferon γ at 500 units/ml, a gift from G. R. Adolf, Ernst-Boehringer-Institut für Arzneimittelforschung, Vienna, Austria) for 24 h before infection) and 105 RF33.70 T–T hybridoma cells, respectively, per well of 96-well plates as described (11, 18, 19). After 24 h of infection/phagocytosis with various multiplicities of infection (moi) and in the presence of OVA (50 μg/ml), macrophages were fixed with 1% paraformaldehyde and appropriate RF33.70 T–T hybridoma cells were added for 24 h as described (11). The stimulation of the hybridomas was quantified accordingly by IL-2-dependent proliferation of CTLL-2 cells (11).
RESULTS
Construction of the Mycobacteria E. coli Shuttle Expression Vectors pAT261:Hly and pMV306:Hly.
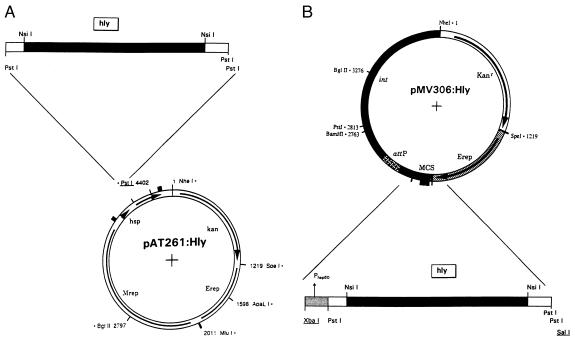
To transfer the phagosomal escape function (mediated by Hly of L. monocytogenes EGD Sv 1/2a, ref. 28), to BCG Chicago, two E. coli–mycobacteria shuttle vectors pAT261 and pMV306 were used. The second-generation vector pAT261, a pMV206 derivative (21), directs extrachromosomal Hly expression with about five plasmid copies per BCG genome and the integrative plasmid pMV306, a precursor of vector pMV361, allows stable chromosomal expression of Hly (Fig. 1) (20).
Figure 1.
Plasmid maps for Hly secretion by r-BCG strains. (A) Extrachromosomal Hly expression by E. coli–mycobacteria shuttle plasmid pAT261:Hly. Insertion of the pILH-1-derived 1.7-kb PstI fragment encoding the DNA sequence of the mature Hly protein. Mrep, mycobacterial replicon; Erep, E. coli origin of replication; kan, kanamycin-resistance gene; hsp, heat shock protein promoter. (B) Chromosomal integrative shuttle vector pMV306:Hly for Hly expression by mycobacteria. The inserted DNA restriction fragment (XbaI–SalI) including the hsp60 promoter is derived from plasmid pAT261:Hly. attP, attachment site of mycobacteriophage L5; MCS, multiple cloning site; int, integrase of mycobacteriophage L5.
A pILH-1-derived 1.7-kb PstI DNA fragment coding for an hly-hlyA (E. coli pHly152-specific hemolysin A) ORF was inserted into PstI site of plasmid pAT261 (15, 20). This resulting gene fusion codes for the expression of secreted proteins directed to the supernatant by the BCG-specific Ag85B signal peptide (29). The construct was termed pAT261:Hly and its XbaI–SalI DNA expression cassette under transcriptional control of the hsp60 mycobacterial promoter was subsequently used for insertion into the parental pMV306 vector resulting in the construct pMV306:Hly (Fig. 1). The DNA sequence of the hly-specific insertion sites in both mycobacterial expression plasmids was analyzed. The derived amino acid sequence of the complete Hly fusion protein is presented in Fig. 2. The mature Hly fusion protein putatively consists of 30 aa at the N terminus and 52 aa at the C-terminal part of the fusion that partially belong to HlyA of E. coli (15).
Figure 2.
Putative amino acid sequence of the Hly fusion protein expressed by BCG pAT261:Hly or BCG pMV306:Hly. The Hly fusion protein consists of the following polypeptide sequences: BCG-specific Ag85B including signal peptide (underlined; previously termed α-antigen in ref. 29); E. coli pHly152-specific HlyA (italic type; ref. 15); mature Hly (boldface type; ref. 28); random amino acid sequence, (roman type). The restriction sites (PstI and NsiI) used for corresponding gene fusions are presented beneath the sequence.
Analysis of Hly Expression in BCG pAT261:Hly and BCG pMV306:Hly.
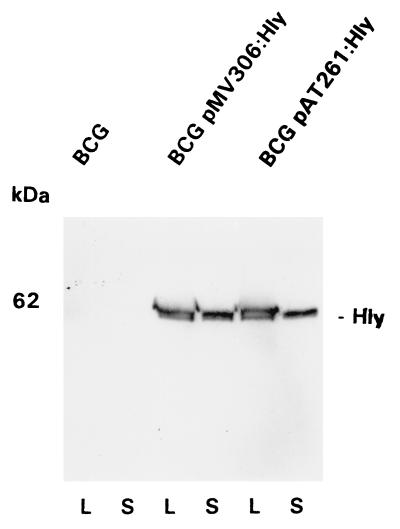
To characterize Hly secretion by the BCG pAT261:Hly or by BCG pMV306:Hly strain appropriate supernatants and mycobacterial lysates of midlogarithmic-grown cultures were prepared according to Stover et al. (20). The Hly fusion protein was detectable in lysates and supernatants of both mycobacterial strains, BCG pAT261:Hly and BCG pMV306:Hly (Fig. 3). The size, 62 kDa, of the Hly-derived polypeptide is slightly larger than that of the original 58-kDa Hly protein of L. monocytogenes.
Figure 3.
Analysis of Hly expression by r-BCG. Detection of Hly fusion protein in lysates (lanes L) or supernatants (lanes S) of BCG, BCG pAT261:Hly, or BCG pMV306:Hly strains by immunostaining. The primary antibody used for chemiluminescent immunostaining of the 62-kDa Hly hybrid protein was anti-Hly mAb H14–3.
Both BCG pAT261:Hly and BCG pMV306:Hly, but not wild-type BCG, expressed hemolytic activity for sheep erythrocytes (Table 1), which proves successful transfer of cytolytic Hly function to mycobacterial species.
Table 1.
Hemolytic activities of supernatant and whole-bacteria suspensions of r-BCG strains and L. monocytogenes EGD
| Strain suspension | Hemolytic activity, complete units
|
|
|---|---|---|
| Supernatant | Whole bacteria | |
| L. monocytogenes EGD | 8 | 16 |
| BCG pAT261:Hly | 2 | 4 |
| BCG pMV306:Hly | 2 | 4 |
| BCG | ND | ND |
Hemolytic activity is given in complete units, which are defined as the reciprocal of the highest dilution of complete hemolysis. The strain suspension contains extracellular and membrane-bound hemolytic activities. ND, nondetectable.
Growth and Localization of r-BCG Strains in Macrophages.
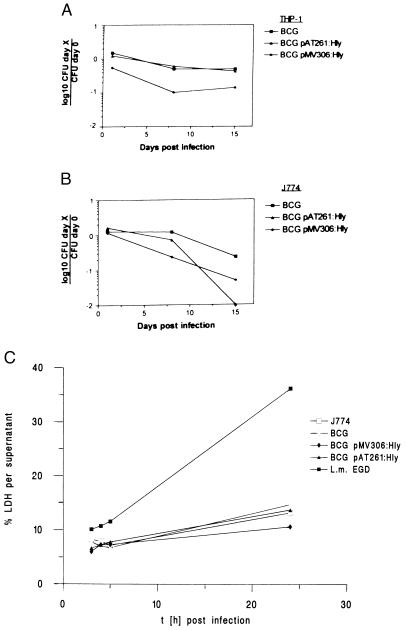
Survival of BCG pAT261:Hly or BCG pMV306:Hly microorganisms in host cells was monitored by determination of mycobacterial colony-forming units from infected macrophages. Three hours after infection of THP-1 or J774A.1 cells at a moi of 10, the efficacy of mycobacterial phagocytosis was determined (Fig. 4). Subsequent long-term culture was performed in the presence of gentamicin (200 μg/ml) to kill released or nonphagocytosed mycobacteria in the supernatant. As depicted in Fig. 4, wild-type BCG persisted virtually at constant numbers, whereas BCG pMV306:Hly bacteria showed impaired intracellular persistence in THP-1 and J774A.1 host cells. Noteworthy, the intracellular survival rate of BCG pMV306:Hly bacteria in THP-1 macrophages was already reduced at day 1 after infection (p.i.) as compared with BCG or BCG pAT261:Hly-infected cells. In addition, viable BCG pAT261:Hly bacteria were not detected in infected J774A.1 cells at day 15 p.i., suggesting sterile elimination of these mycobacterial constructs at least in the presence of gentamicin.
Figure 4.
Intracellular growth and cytotoxicity of r-BCG strains. (A) Survival of wild-type BCG (▪), BCG pAT261:Hly (▴), and BCG pMV306:Hly (⧫) strains in human macrophage-like cells THP-1. (B) Survival of wild-type BCG (▪), BCG pAT261:Hly (▴), and BCG pMV306:Hly (⧫) strains in murine J774A.1 macrophage-like cells. Colony-forming units were determined from infected macrophages from 3 h to 15 days p.i. The data are presented as means ± SD (n = 3). (C) Supernatants and cell lysates of J774A.1 were assayed for LDH activity after BCG or r-BCG infection. J774A.1 (□), BCG (⋄), BCG pMV306:Hly (⧫), BCG pAT261:Hly (▪), or L. monocytogenes EGD (▪). The cumulative percentage of total LDH activity detected in the supernatant (mean ± SD) is given. This is a representative experiment of three. The percent LDH released into the supernatant was determined as a measure of cell death.
To gain insights into the reasons for impaired intracellular persistence of BCG pAT261:Hly and BCG pMV306:Hly strains, their cytotoxicity for J774A.1 macrophages was determined in short-term cultures at a moi of 10. Cytotoxicity was analyzed by measuring LDH activity in supernatants of host cells infected with BCG, BCG pAT261:Hly, BCG pMV306:Hly, or L. monocytogenes EGD at 3, 4, 5 and 24 h p.i. (Fig. 4C). At 24 h p.i., the amount of released LDH into supernatants did not significantly differ among parental BCG-, BCG pAT261:Hly-, and BCG pMV306:Hly-infected and noninfected host cells. In contrast, the fast-growing and hemolytic L. monocytogenes EGD strain caused profound LDH release into the supernatant within 24 h p.i. (Fig. 4C). These data suggest that r-BCG strains secreting Hly did not express cytotoxic activity for macrophages. Rather, Hly secretion impaired persistence of BCG in macrophages. Thus, Hly reduced persistence of BCG, at least in vitro.
Immunocytochemical Localization of Hly in r-BCG-Infected Macrophages.
Immunoelectron microscopic analysis of macrophages infected with wild-type BCG revealed that the bacilli were retained in vacuoles, the majority of which labeled with anti-LAMP-1 antibodies (Fig. 5). Examination of macrophages infected with BCG pAT261:Hly or BCG pMV306:Hly revealed a similar intraphagosomal distribution of bacilli. Labeling with affinity-purified rabbit anti-Hly antibody demonstrated synthesis and release of Hly from the intracellular bacteria, although the bacilli harboring the Hly-encoding constructs did not escape from their vacuoles. The Hly signal was not only observed in bacilli and the surrounding luminal space but also in small vesicles distinct from the bacteria-containing vacuoles. Communication between bacterial phagosomes and the endosomal continuum has been reported previously on basis of lipoarabinomannan distribution in M. tuberculosis and Mycobacterium avium-infected macrophages (30).
Figure 5.
Immunoelectron micrographs of murine bone marrow-derived macrophages infected with BCG pMV306:Hly or BCG pAT261:Hly. BCG pMV306:Hly (a) or BCG pAT261:Hly infection (b) were probed with affinity-purified rabbit anti-Hly (goat anti-rabbit IgG + 18-nm gold) and rat mAb ID4B against murine LAMP-1 (goat anti-rat IgG + 6-nm gold) 24 h p.i. Labeling with anti-LAMP-1 antibody is indicated by arrowheads. (Bars = 0.25 μm.)
MHC Class I Presentation of OVA by r-BCG-Infected Macrophages.
Hly promotes egression of L. monocytogenes organisms from the phagosome to the cytosol of host cells, thus facilitating MHC class I processing of listerial antigens. We determined MHC class I presentation of OVA in BM A3.1A7 macrophage-like cells by simultaneous incubation with OVA (50 μg/ml) and BCG pAT261:Hly or BCG pMV306:Hly or parental BCG microorganisms at various moi.
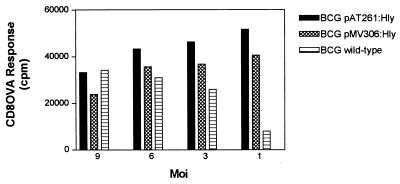
Codelivery of OVA in interferon-γ-activated and mycobacteria-infected macrophages revealed that BCG pAT261:Hly and BCG pMV306:Hly microorganisms facilitated access of OVA to the MHC class I presentation pathway as indicated by MHC class I-specific OVA257–264 epitope recognition by T–T hybridoma cells RF33.70 (Fig. 6). The stimulation of OVA-specific CD8 T cell hybridomas was equally promoted by the BCG pAT261:Hly or BCG pMV306:Hly strain at higher moi of 9 to 3, as it was by the wild-type BCG. At a low moi of 1, however, BCG wild-type failed to promote MHC class I processing of OVA, whereas both r-BCG pAT261:Hly and BCG pMV306:Hly strains were still capable of promoting pronounced MHC class I processing. Thus, Hly secreted by BCG pAT261:Hly or BCG pMV306:Hly generated a route for more efficient MHC class I delivery of soluble antigens.
Figure 6.
Presentation of OVA to CD8 T cells by MHC class I molecules of macrophages infected with BCG pAT261:Hly, BCG pMV306:Hly, or BCG wild-type microorganisms. Interferon-γ-activated and BM A3.1A7 cells (H-2Kb haplotype) were incubated with BCG pAT261:Hly (solid bars), BCG pMV306:Hly (cross-hatched bars), or BCG wild-type (horizontal hatched bars) bacteria in presence of OVA (50 μg/ml) at the indicated moi for 24 h, fixed with 1% paraformaldehyde, washed, and subsequently cocultured with OVA257–264-specific CD8 T–T hybridoma cells RF33.70 for 24 h. Supernatants were assayed for IL-2. Data are presented in triplicate from a representative experiment; the SEM never exceeded 10%. Noninfected BM A3.1A7 macrophages with or without OVA coculture never exceeded 4,000 cpm. Experiments were performed three times with similar results.
DISCUSSION
The present report describes the construction of r-M. bovis BCG strains that secrete the cytolytic Hly of L. monocytogenes. (i) Supernatants of BCG pAT261:Hly or BCG pMV306:Hly caused hemolysis of erythrocytes, showing that Hly was expressed in biologically active form by r-BCG. (ii) These r-BCG microorganisms with Hly secretion apparently failed to escape from the phagosome of macrophages. (iii) The association between Hly and the vacuolar membrane of infected macrophages may reflect binding to its putative cholesterol receptor. (iv) Hly fusion proteins secreted by r-BCG within the vacuole augmented MHC class I presentation of an unrelated protein antigen. (v) Intracellular host cell persistence of BCG pAT261:Hly or BCG pMV306:Hly was reduced in comparison to the parental wild-type BCG carrier.
It has been reported that M. tuberculosis H37Rv and H37Ra strains express contact-dependent cytolytic activity (31). Although such cytolytic activities could be responsible for phagosomal escape of M. tuberculosis into the cytosol of macrophages described by McDonough et al. (32), convincing evidence exists that M. tuberculosis remains in the phagosome (33, 34). Recently, Henderson et al. (35) detected occasional breaks in the multimembrane of M. tuberculosis-containing phagosomes in dendritic cells. BCG bacteria do not express cytolytic activity and have not been observed escaping from their intracellular vacuoles (31, 32).
In an attempt to imbue the vaccine strain BCG with the ability to lyse their phagosomal membrane and access the cell cytosol, BCG strains were constructed that expressed the Hly lysin of L. monocytogenes. Secretion of the Hly hybrid by BCG pAT261:Hly or BCG pMV306:Hly strain was achieved via plasmid expression or chromosomal insertion, respectively. Both r-strains BCG pAT261:Hly and BCG pMV306:Hly secreted hemolytic activity at pH 6. In a previous study, an analogous Hly fusion derivative secreted by Salmonella dublin aroA was active at acidic pH (15). This r-Salmonella strain was capable of leaving the phagosomal compartment of J774A.1 macrophage-like cells (15). In contrast, BCG pAT261:Hly and BCG pMV306:Hly microorganisms apparently did not escape from the phagosome into the cytosol of macrophages. This failure of endosomal release of r-BCG microorganisms could have several reasons: (i) It is possible that the endosomal pH of r-BCG-containing vacuoles were suboptimal for the biological activity of Hly. (ii) Binding of the Hly monomer to its putative cholesterol receptor in the endosomal membrane or subsequent oligomerization of monomers could be disturbed by putative conformational alterations of the Hly fusion protein (36).
The basis for the pH optimum of Hly remains unknown, as yet. Except for studies in which various residues of the conserved cysteine-containing undecapeptide had been altered, residues that may contribute to the pH optimum of Hly have not been identified (36). In general, Hly activity has an optimum at acidic pH 5.5, the approximate pH that exists in the phagolysosome after its formation. Minor cytolytic activity of Hly is detected at a pH that is at or above 7 (36). Previous studies on the pH of M. avium-containing vacuoles indicated that they were only mildly acidic, equilibrating to pH 6.2 to 6.5 (37). Recent findings of BCG-containing vacuoles up to 2 h p.i. revealed a similar range of pH values (D.G.R., unpublished results). As a corollary, the Hly fusion protein secreted by r-BCG pAT261:Hly or BCG pMV306:Hly may still be active in phagosomes carrying mycobacteria.
In general, maturation along the endosomal–lysosomal pathway in mycobacteria-containing macrophages is best viewed as occurring along a continuum with progressive acquisition of cathepsin D in its immature form and LAMPs (e.g., LAMP-1) as a result of multiple vesicular fusion events (37, 38). Immunogold-staining of Hly fusion proteins secreted by BCG pAT261:Hly revealed that Hly was consistently associated with membranes of the endosomal/lysosomal continuum, as evidenced by the presence of the endosomal marker LAMP-1 on these membranes. These data suggest that the Hly conformation allows its binding to cholesterol receptors in phospholipid bilayers of intracellular vesicles.
Although BCG constructs secreting Hly apparently did not leave the phagosome, we assume that Hly formed pores that promoted translocation of secreted proteins from the phagosome into the cytoplasm of antigen-presenting cells. Our data of increased OVA delivery to the MHC class I presentation pathway in macrophages infected with BCG pAT261:Hly or BCG pMV306:Hly support this notion. By means of these pores, a route for MHC class I presentation of OVA and possibly mycobacterial antigens could be generated. Thus, at a low moi, MHC class I presentation of the immunodominant OVA257–264 epitope by BCG pAT261:Hly- or BCGpMV306:Hly-infected macrophages was significantly higher than that afforded by wild-type BCG. We conclude that BCG pAT261:Hly and BCG pMV306:Hly facilitate introduction of soluble mycobacterial antigens into the MHC class I presentation pathway and thus promote recognition of infected macrophages by CD8 T cells.
Phagocytosis of M. tuberculosis, but not of wild-type BCG, by BM A3.1A7 macrophages in presence of OVA promoted introduction of the immunodominant OVA257–264 epitope into the MHC class I presentation pathway at the low moi of 3 (11). Yet, similar to our experiment at a higher moi, wild-type BCG was capable of introducing OVA into the MHC class I pathway though at low efficiency (11). Recent studies reported that the cytolytic activity of soluble Hly generates a route for the presentation of OVA by MHC class I molecules (39). Consistent with these findings, the direct transfer of the cytolysin Hly to BCG improved the capacity of this vaccine to efficaciously stimulate MHC class I-restricted CD8 T cells. The central role of CD8 T cells in protection against TB has been established (7). In contrast, CD8 T cells are poorly stimulated by BCG (7). This discrepancy between requirement of CD8 T cells for protection and inadequate stimulation of CD8 T cells by BCG has been considered a reason for the unsatisfactory vaccine efficacy of BCG against TB. Our finding that Hly-secreting r-BCG strains promote MHC class I processing of soluble antigens without expressing increased in vitro persistence reveal a strategy for overcoming this drawback. Future experiments should be directed at clarifying whether these r-BCG strains possess increased vaccine efficacy against TB in experimental animal models.
Acknowledgments
We thank M. Hess and Dr. P. Conradt for helpful discussions. We are grateful to Drs. P. Cossart and D. Portnoy for donating antibodies directed against Hly. Many thanks to Drs. I. Gentschev and W. Goebel, to MedImmune and Dr. William R. Jacobs, Jr., to Drs. G. R. Adolf and M. K. Gately for providing plasmid pILH-1 and plasmids pAT261 and pMV306 and r-cytokines, respectively. Furthermore, we thank Dr. K. L. Rock for providing the Ova-specific T–T hybridoma cells RF33.70 and macrophage-like cell line BM A3.1A7. This work was supported by the German Science Foundation, project Ka 573/3–1/2 to S.H.E.K. and J.H. and the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie project “Mycobacterial Infections.” D.G.R. is supported by U.S. Public Health Service Grants HL 55936 and AI 33348.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: BCG, Bacille Calmette-Guérin; TB, tuberculosis; r-, recombinant; moi, multiplicity of infection; p.i., after infection; LAMP, lysosome-associated membrane glycoprotein; MHC, major histocompatibility complex; OVA, ovalbumin; IL, interleukin.
References
- 1.Kochi A. Tubercle. 1991;72:1–6. doi: 10.1016/0041-3879(91)90017-m. [DOI] [PubMed] [Google Scholar]
- 2.Coldlitz G A, Brewer T F, Berkey C S, Wilson M E, Burdick E, Fineberg H V, Mosteller F. J Am Med Assoc. 1994;271(9):698–702. [PubMed] [Google Scholar]
- 3.Roche P W, Triccas J A, Winter N. Trends Microbiol. 1995;3:397–401. doi: 10.1016/s0966-842x(00)88986-6. [DOI] [PubMed] [Google Scholar]
- 4.Murray P J, Aldovini A, Young R A. Proc Natl Acad Sci USA. 1996;93:934–939. doi: 10.1073/pnas.93.2.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hess J, Kaufmann S H E. FEMS Microbiol Immunol. 1993;7:95–103. doi: 10.1111/j.1574-695X.1993.tb00387.x. [DOI] [PubMed] [Google Scholar]
- 6.Kaufmann S H E. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 7.Kaufmann S H E. In: Fundamental Immunology. Paul W E, editor. New York: Raven; 1997. , in press. [Google Scholar]
- 8.Berche P, Gaillard J L, Sansonetti P J. J Immunol. 1987;138:2266–2276. [PubMed] [Google Scholar]
- 9.Flynn J L, Goldstein M M, Triebold K J, Koller B, Bloom B R. Proc Natl Acad Sci USA. 1992;89:12013–12017. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ladel C H, Daugelat S, Kaufmann S H E. Eur J Immunol. 1995;25:377–384. doi: 10.1002/eji.1830250211. [DOI] [PubMed] [Google Scholar]
- 11.Mazzaccaro R Z, Gedde M, Jensen E R, Van Santen H M, Ploegh H L, Rock K L, Bloom B R. Proc Natl Acad Sci USA. 1996;93:11786–11791. doi: 10.1073/pnas.93.21.11786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Portnoy D A, Jacks P S, Hinrichs D J. J Exp Med. 1988;167:1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaillard J L, Berche P, Mounier J, Richard S, Sansonetti P J. Infect Immun. 1987;55:2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bielecki J, Youngman P, Connelly P, Portnoy D A. Nature (London) 1990;354:175–176. doi: 10.1038/345175a0. [DOI] [PubMed] [Google Scholar]
- 15.Gentschev I, Sokolovic Z, Mollenkopf H-J, Hess J, Kaufmann S H E, Kuhn M, Krohne G F, Goebel W. Infect Immun. 1995;63:4202–4205. doi: 10.1128/iai.63.10.4202-4205.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hess J, Kaufmann S H E. In: Host Response to Intracellular Pathogens. Kaufmann S H E, editor. Austin, TX: Landes; 1997. pp. 75–90. [Google Scholar]
- 17.Hess J, Gentschev I, Miko D, Welzel M, Ladel C, Goebel W, Kaufmann S H E. Proc Natl Acad Sci USA. 1996;93:1458–1463. doi: 10.1073/pnas.93.4.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rock K L, Rothstein L, Gamble S. J Immunol. 1990;145:804–811. [PubMed] [Google Scholar]
- 19.Kovacsovics-Bankowski M, Clark K, Benacerraf B, Rock K L. Proc Natl Acad Sci USA. 1993;90:4942–4946. doi: 10.1073/pnas.90.11.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stover C K, Bansal G P, Hanson M S, Burlein J E, Palaszynski S R, Young J F, Koenig S, Young D B, Sadziene A, Barbour A G. J Exp Med. 1993;178:197–209. doi: 10.1084/jem.178.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stover C K, de la Cruz V F, Fuerst T R, Burlein J E, Benson L A, Bennett L T, Bansal G P, Young J F, Lee M H, Hatfull G F, et al. Nature (London) 1991;351:456–460. doi: 10.1038/351456a0. [DOI] [PubMed] [Google Scholar]
- 22.Schoel B, Welzel M, Kaufmann S H E. J Chromatogr A. 1994;667:131–139. doi: 10.1016/0021-9673(94)89061-7. [DOI] [PubMed] [Google Scholar]
- 23.Nato F, Reich K, Lhopital S, Rouye S, Geoffroy C, Mazie J C, Cossart P. Infect Immun. 1991;59:4641–4646. doi: 10.1128/iai.59.12.4641-4646.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reyrat J M, Lopez-Ramirez G, Ofredo C, Gicquel B, Winter N. Infect Immun. 1996;64:3934–3936. doi: 10.1128/iai.64.9.3934-3936.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogers H W, Callery M P, Deck B, Unanue E R. J Immunol. 1996;156:679–684. [PubMed] [Google Scholar]
- 26.Russell D G, Xu S, Chakraborty P. J Cell Sci. 1992;103:1193–1210. doi: 10.1242/jcs.103.4.1193. [DOI] [PubMed] [Google Scholar]
- 27.Russell D G. In: Methods in Cell Biology. Russell D G, editor. London: Academic; 1994. pp. 277–288. [Google Scholar]
- 28.Domann E, Chakraborty T. Nucleic Acids Res. 1989;17:6406. doi: 10.1093/nar/17.15.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuo K, Yamaguchi R, Yamazaki A, Tasaka H, Terasaka K, Yamada T. J Bacteriol. 1990;170:3847–3854. doi: 10.1128/jb.170.9.3847-3854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu S, Cooper A, Sturgill-Koszycki S, van Heyningen T, Chatterjee D, Orme I, Allen P, Russell D G. J Immunol. 1994;153:2568–2577. [PubMed] [Google Scholar]
- 31.King C H, Mundayoor S, Crawford J T, Shinnik T M. Infect Immun. 1993;61:2708–2712. doi: 10.1128/iai.61.6.2708-2712.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDonough K A, Kress Y, Bloom B R. Infect Immun. 1993;61:2763–2773. doi: 10.1128/iai.61.7.2763-2773.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russell D G. Trends Cell Biol. 1995;5:125–128. doi: 10.1016/s0962-8924(00)88963-1. [DOI] [PubMed] [Google Scholar]
- 34.Horwitz M A. The Immunologist. 1997;5/1:15–20. [Google Scholar]
- 35.Henderson R A, Watkins S C, Flynn J L. J Immunol. 1997;159:635–643. [PubMed] [Google Scholar]
- 36.Tweten R K. In: Virulence Mechanisms of Bacterial Pathogens. Roth J A, Bolin C A, Brogden K A, Minion F C, Wannemuehler M J, editors. Washington, DC: Am. Soc. Microbiol.; 1995. pp. 207–228. [Google Scholar]
- 37.Sturgill-Koszycki S, Schlesinger P H, Chakraborty P, Haddix P L, Collins H L, Fok A K, Allen R D, Gluck S L, Heuser J, Russell D G. Science. 1994;263:678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- 38.Sturgill-Koszycki S, Schaible U E, Russell D G. EMBO J. 1996;15:6960–6968. [PMC free article] [PubMed] [Google Scholar]
- 39.Darji A, Chakraborty T, Wehland J, Weiss S. Eur J Immunol. 1996;25:2967–2971. doi: 10.1002/eji.1830251038. [DOI] [PubMed] [Google Scholar]