Abstract
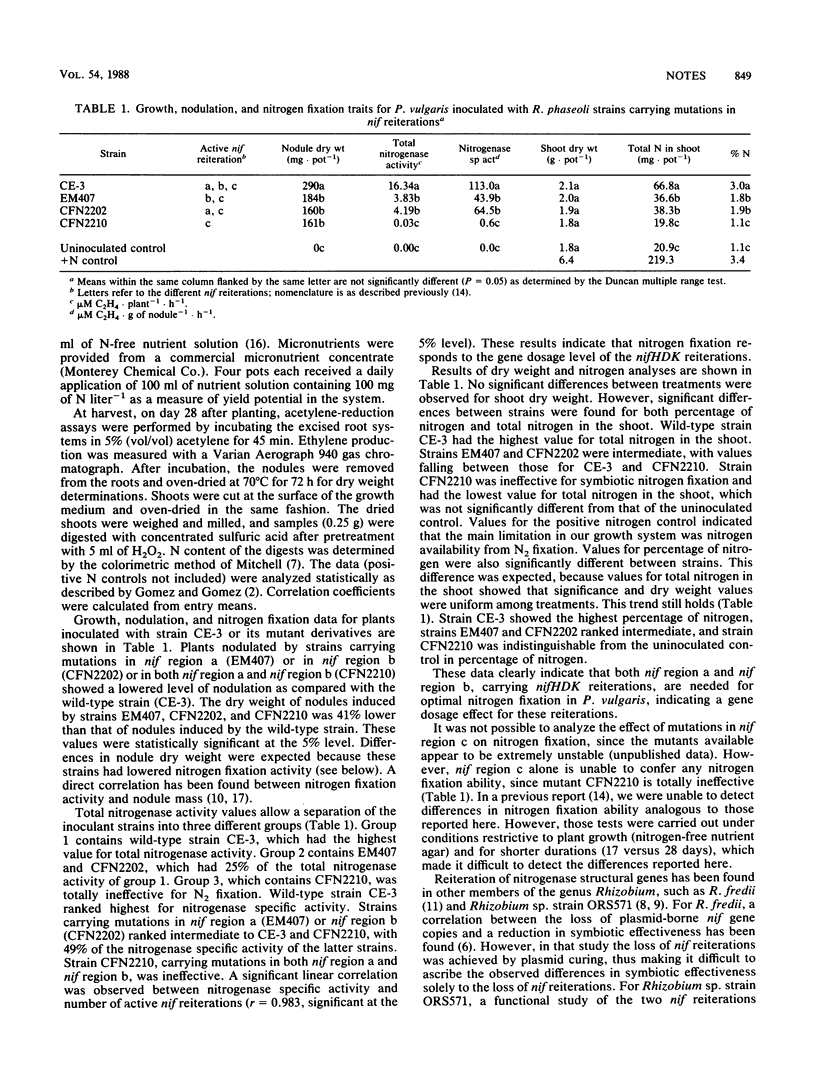
Most naturally occurring strains of Rhizobium phaseoli possess reiteration of the nif genes. Three regions contain nitrogenase structural genes in strain CFN42. Two of these regions (a and b) have copies of nifH, nifD, and nifK, whereas the third region (c) contains only nifH. Strains containing mutations in either nif region a or nif region b had significantly diminished symbiotic effectiveness compared with the wild-type strain on the basis of nodule mass, total nitrogenase activity per plant, nitrogenase specific activity, total nitrogen in the shoot, and percentage of nitrogen. A strain containing mutations in both nif region a and nif region b was totally ineffective. These data indicate that both nif region a and nif region b are needed for full symbiotic effectiveness in R. phaseoli.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Corbin D., Barran L., Ditta G. Organization and expression of Rhizobium meliloti nitrogen fixation genes. Proc Natl Acad Sci U S A. 1983 May;80(10):3005–3009. doi: 10.1073/pnas.80.10.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göttfert M., Horvath B., Kondorosi E., Putnoky P., Rodriguez-Quiñones F., Kondorosi A. At least two nodD genes are necessary for efficient nodulation of alfalfa by Rhizobium meliloti. J Mol Biol. 1986 Oct 5;191(3):411–420. doi: 10.1016/0022-2836(86)90136-1. [DOI] [PubMed] [Google Scholar]
- Leemans J., Deblaere R., Willmitzer L., De Greve H., Hernalsteens J. P., Van Montagu M., Schell J. Genetic Identification of functions of TL-DNA transcripts in octopine crown galls. EMBO J. 1982;1(1):147–152. doi: 10.1002/j.1460-2075.1982.tb01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis J. N., Barbour W. M., Elkan G. H. Effect of Sym Plasmid Curing on Symbiotic Effectiveness in Rhizobium fredii. Appl Environ Microbiol. 1985 Jun;49(6):1385–1388. doi: 10.1128/aem.49.6.1385-1388.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash R. K., Atherly A. G. Reiteration of genes involved in symbiotic nitrogen fixation by fast-growing Rhizobium japonicum. J Bacteriol. 1984 Nov;160(2):785–787. doi: 10.1128/jb.160.2.785-787.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinto C., De La Vega H., Flores M., Leemans J., Cevallos M. A., Pardo M. A., Azpiroz R., De Lourdes Girard M., Calva E., Palacios R. Nitrogenase reductase: A functional multigene family in Rhizobium phaseoli. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1170–1174. doi: 10.1073/pnas.82.4.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton P. W., Tavares J. W. Inoculation response of legumes in relation to the number and effectiveness of indigenous Rhizobium populations. Appl Environ Microbiol. 1986 May;51(5):1013–1018. doi: 10.1128/aem.51.5.1013-1018.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soberón-Chávez G., Nájera R., Olivera H., Segovia L. Genetic rearrangements of a Rhizobium phaseoli symbiotic plasmid. J Bacteriol. 1986 Aug;167(2):487–491. doi: 10.1128/jb.167.2.487-491.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


