Abstract
Magnetic resonance imaging was used to measure the hippocampal and amygdalar volumes of 60 chimpanzees (Pan troglodytes). An asymmetry quotient (AQ) was then used to calculate the asymmetry for each of the structures. A one-sample t test indicated that there was a population-level right hemisphere asymmetry for the hippocampus. There was no significant population-level asymmetry for the amygdala. An analysis of variance using sex and rearing history as between-group variables showed no significant main effects or interaction effects on the AQ scores; however, males were more strongly lateralized than females. Several of these findings are consistent with results found in the human literature.
It has been well documented that humans exhibit neuroanatomical asymmetries in cortical and subcortical brain areas (Bradshaw & Rogers, 1993; Corballis, 1992; Hellige, 1993; Toga & Thompson, 2003). Some have suggested that neuroanatomical and functional asymmetries are unique to humans (Crow, 1998), but recent studies have shown asymmetries in the brains of nonhuman species (Rogers & Andrew, 2002). In nonhuman primates, initial reports of asymmetries focused on differences in the shape of the skull (i.e., petalias), with the great apes exhibiting a left occipital, right frontal asymmetry (Holloway & De La Coste-Lareymondie, 1982; Hopkins & Marino, 2000; LeMay, 1976; Pilcher, Hammock, & Hopkins, 2001). More recently, in nonhuman primates, measures of neuroanatomical asymmetries from cadaver specimens and magnetic resonance imaging (MRI) have reported left hemisphere biases in the sylvian fissure (Hopkins, Pilcher, & MacGregor, 2000; Yeni-Komshian & Benson, 1976), planum temporale (Cantalupo, Pilcher, & Hopkins, 2003; Gannon, Holloway, Broad-field, & Braun, 1998; Gilissen, 2001; Hopkins, Marino, Rilling, & MacGregor, 1998), precentral gyrus (Hopkins & Pilcher, 2001), and a portion of the inferior frontal lobe corresponding to a portion of Broca's area (Cantalupo & Hopkins, 2001). In addition, the cerebellum of great apes has shown developmental torque, or a change in the direction of asymmetry, along the anterior–posterior axis (Cantalupo, Freeman, & Hopkins, in press). Developmental torque has also been found in humans, although in a different pattern than is seen in great apes (Snyder, Bilder, Wu, Bogerts, & Lieberman, 1995).
One aspect of cerebral lateral organization that has not been investigated in great apes is the limbic system, in particular the hippocampus, which has been linked to the formation of memories (Abe, 2001; Cahill & McGaugh, 1998), and amygdala, which has been shown to play a role in emotions such as fear (Aggleton, 1992, 2000; LeDoux, 1993). Behavioral studies in marmosets (Hook-Costigan & Rogers, 1998), rhesus monkeys (Hauser, 1993), and chimpanzees (Fernandez-Carriba, Loeches, Morcillo, & Hopkins, 2002) have revealed left hemiface (right hemisphere) asymmetries in the production of facial expressions. This suggests that aspects of the limbic system may be lateralized to the right hemisphere and possibly linked to asymmetries in the rest of the brain.
Studies in human subjects have indicated that, in most cases, the hippocampus is larger in the right hemisphere (Bilir et al., 1998; Free et al., 1995; Hasboun et al., 1996; Pegues, Rogers, Amend, Vinogradov, & Deicken, 2003; Watson et al., 1992), although in some cases no asymmetry was reported (Bhatia, Bookheimer, Gaillard, & Theodore, 1993; Reiman et al., 1998; Strakowski et al., 1999). Asymmetries in the amygdala have been less reliable, with no asymmetry reported by several researchers (Mori et al., 1997; Soininen et al., 1994; Strakowski et al., 1999), whereas others have reported a larger amygdala in the right hemisphere (Watson et al., 1992). In light of the evidence of behavioral asymmetries in facial expressions and right hemisphere advantages in visual–spatial discrimination (Hopkins & Morris, 1989) and spatial memory (Hopkins & Washburn, 1994; Jason, Cowey, & Weiskratz, 1984) in nonhuman primates, we hypothesized that right hemisphere asymmetries would be evident in the hippocampus and amygdala.
In addition to the basic descriptive data on asymmetries in the amygdala and hippocampus, we examined the potential influence of sex, rearing history, and age of the subjects on overall volume and asymmetries of these two structures. Studies in the human and nonhuman primate literature have reported conflicting results when looking at the associations between volume and asymmetries of the hippocampus and amygdala, with respect to age and sex. In humans, two studies looking at aging and the volume of the hippocampus found no significant association (Bigler et al., 1997; Sheline, Sanghavi, Mintun, & Gado, 1999), whereas Scahill et al. (2003) found that the hippocampus decreased in volume with increasing age. In terms of sex differences, two studies reported no difference between males and females in hippocampus and amygdala volumes (rhesus monkeys: Franklin et al., 2000; humans: Gur, Gunning-Dixon, Bilker, & Gur, 2002). One study found that women have a larger hippocampal volume (Agartz, Momenan, Rawlings, Kerich, & Hommer, 1999; Bigler et al., 1997), whereas a fourth study found that the amygdala was larger in boys (Durston et al., 2001). Part of the discrepancy in sex differences may reflect differences in subject characteristics.
Lastly, previous studies in differentially reared monkeys have not found significant differences in the volume of the hippocampus and amygdala (Lyons, Yang, Sawyer-Glover, Moseley, & Schatzberg, 2001; Sanchez, Hearn, Do, Rilling, & Herndon, 1998). Notwithstanding, rearing history does have a significant influence on laterality in rodents (see Denenberg, 1988), and we sought to examine whether a similar pattern of results might be evident in chimpanzees.
To test this hypothesis, we obtained MRI scans in a sample of chimpanzees. From the MRI scans, the left and right amygdala and hippocampus were traced and measures of volume and asymmetries were calculated. We also evaluated whether there were significant associations between hippocampal and amygdalar asymmetries and the age, sex, and rearing history of the subjects.
Method
Subjects
MRI scans were collected in a sample of 60 chimpanzees (Pan troglodytes), including 32 females and 28 males, ranging in age from 8 to 48 years (M = 22.07, SD = 11.63). All the chimpanzees were members of a captive colony housed at Yerkes National Primate Research Center (YNPRC) in Atlanta, Georgia. Ten apes were wild-caught, 15 were raised by their mothers, and the remaining 35 were human-raised. Fifteen of the brains were scanned post mortem, whereas the other 45 subjects were alive and healthy at the time of the scan. Ages ranged from 11 to 48 years (M = 26.60 years) for the post mortem brains and 8 to 47 years (M = 20.20 years) for the in vivo scans.
MRI Procedure
Subjects were first immobilized by ketamine injection (2–6 mg/kg) and subsequently anesthetized with propofol (10 mg−1 · kg−1 · hr−1), following standard procedures, at the YNPRC. Subjects were then either transported to the MRI facility at Emory University Hospital or to a portable MRI scanner at YNPRC. The subjects remained anesthetized for the duration of the scans and for the time needed to travel between YNPRC and Emory Hospital (total time ∼ 2 hr) or between their enclosure and the MRI portable scanner (total time ∼ 1 hr). At the MRI facility, the living apes were placed in the scanner chamber in a supine position with their heads fitted inside the human-head coil. The cadaver brains were placed inside the human-knee coil with the dorsal side up. Scan duration ranged between 40 and 80 min as a function of brain size. This project involved the use of two MRI machines (Phillips, Model 51), each with 1.5-Tesla superconducting magnets. For all subjects, T1-weighted images were collected in the transverse plane using a gradient echo protocol (pulse repetition = 19.0 ms, echo time = 8.5 ms, number of signals averaged = 8, matrix = 256 × 256). These scan parameters were developed in previous studies (Hopkins et al., 1998, 2000) and provided good resolution of the brain areas of interest to this study. After completing MRI procedures, the subjects were returned to the YNPRC and temporarily housed in a single cage for 6 –12 hr to allow the effects of the anesthesia to wear off, after which they were returned to their home cages. The archived MRI data were stored on optical disks, and the collected images were realigned and sliced into coronal slices with ANALYZE software (Biomedical Imaging Resource; Mayo Foundation, Rochester, MI).
Amygdalar Area
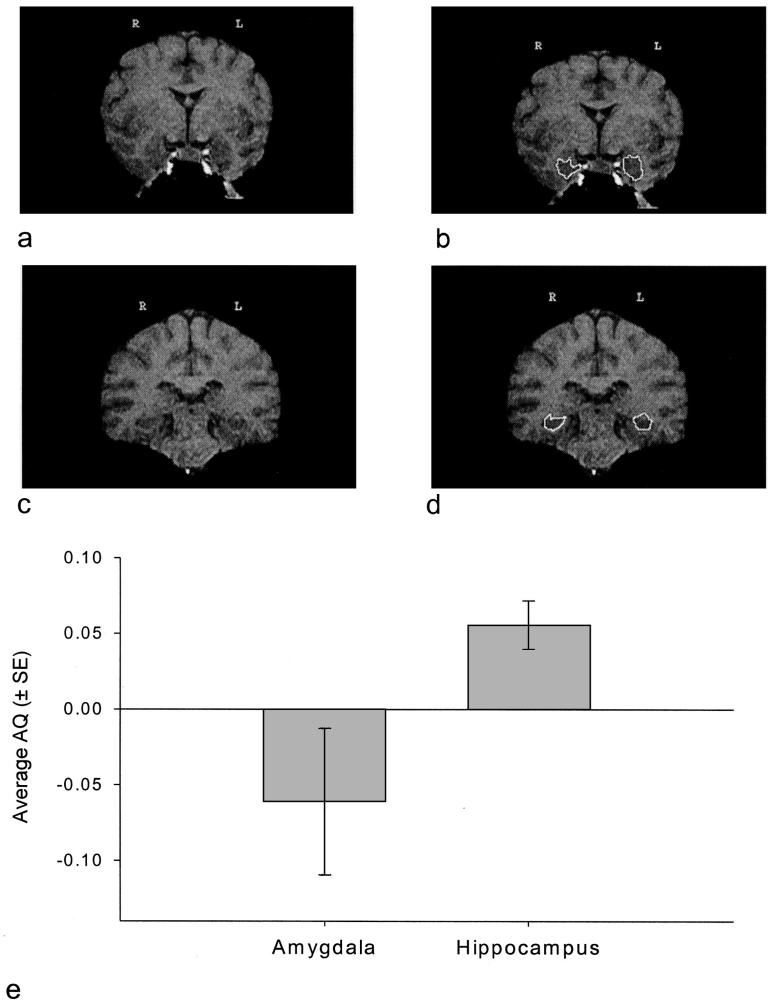
The MRI scans taken of the entire brain were aligned in the sagittal, coronal, and axial planes according to standard anatomical landmarks and cut into 1-mm coronal slices with multiplanar reformatting software (ANALYZE). The regions of interest in each coronal slice were measured (in square millimeters) with a mouse-driven computer-guided area tool available in ANALYZE. The methodology used for identifying the amygdala was adapted from human protocols (Bilir et al., 1998; Brierley, Shaw, & David, 2002). Coronal slices were used in order to assess the volume of the amygdala. Figure 1a shows a coronal slice that includes the amygdala; Figure 1b shows the same coronal slice with the amygdala outlined at defined boundaries. The anterior border was defined as the slice immediately posterior to where the optic chiasm first appeared continuous. The posterior border and separation of the amygdala from the hippocampus were defined as the slice anterior to where the inferior horn of the lateral ventricle was first visible. In addition, the most anterior slice usually showed at least one cerebral artery. Although the medial border of the amygdala in humans is usually the uncal notch, it was not clear enough in the great ape scans to be used as a landmark. Instead, the medial border included all gray matter in the medial temporal lobe, including the ambient gyrus. The lateral border was defined by the white matter of the temporal stem. The entorhinal sulcus was used as a superior border for the amygdala.
Figure 1.
Coronal slices of in vivo chimpanzee magnetic resonance imaging scan including amygdala (a), amygdala traced at defined boundaries (b), hippocampus (c), and hippocampus traced at defined boundaries (d). e: Average (± SE) asymmetry quotient (AQ) values for the amygdala and hippocampus.
Hippocampal Area
Landmarks used for defining the hippocampus were adapted from protocols used with human subjects (De Bellis et al., 2000; Gosche, Mortimer, Smith, Markesbery, & Snowdon, 2001; Massana et al., 2003, Pegues et al., 2003; Szabo et al., 2001). A coronal view of the entire brain was used to measure the volume of the hippocampus. Figure 1c shows a coronal slice that includes the hippocampus; Figure 1d shows the same coronal slice with the hippocampus outlined at defined boundaries. The anterior border of the hippocampus was defined as the first slice in which the inferior horn of the lateral ventricle was visible. The posterior border was the slice anterior to the crus of the fornix. This excluded the isthmus of the cingulate gyrus and the parahippocampal gyrus. The medial border of the hippocampus was defined by white matter represented by the alveus, or the ambient cistern if the alveus could not be seen. The ambient cistern and alveus were often difficult to distinguish from the hippocampus; in these cases, several tactics were used, including enlarging the image of the slice to try and distinguish white matter and comparing anterior and posterior slices to determine whether white matter was present. Laterally, the hippocampal border was the temporal horn of the lateral ventricle. The white matter of the parahippocampal gyrus was used as the inferior border. The choroid fissure was used as the superior border of the hippocampus. The definition of the hippocampus included the cornus ammonis, dentate gyrus, subiculum, subicular complex, and hippocampus proper. The fimbria and alveus were not included in the measurement of the hippocampus. Because the delineation of the amygdala from the hippocampus was only based on the inferior horn of the lateral ventricle, rather than the alveus, some of the amygdala may have been included in hippocampus measurements.
Data Analysis
For each brain area, an asymmetry quotient (AQ) was calculated according to the formula (R − L) ÷ [(R + L) × 0.5]. The sign of the resulting value indicated the direction of asymmetry (positive value = right hemisphere bias, negative value = left hemisphere bias). The absolute AQ values (ABS-AQ) reflected the magnitude of asymmetry. The hippocampal volume was calculated by adding the sum of the areas of each of the hippocampal slices in the left hemisphere to the sum of the areas of the hippocampal slices in the right hemisphere. The same procedure was used to calculate the amygdalar volume, using the area for each amygdalar slice. Lastly, the sum of both the left and right amygdala and hippocampus was calculated and an AQ was derived for these values by means of the formula described above.
The interrater reliability of two investigators was examined with the Pearson product moment correlation for the asymmetry of the hippocampus and amygdala on six scans, including two cadaver specimens and four in vivo brain scans. For the amygdala, interrater reliability coefficients were low and nonsignificant for the left, r(35) = .28, and right, r(35) = .40, sides. In terms of the hippocampus, interrater reliability coefficients for the left, r(121) = .68, p < .05, and right, r(121) = .72, p < .05, sides were significant. The intrarater reliability was also calculated from the same six scans used for interrater reliability after all the scans were traced. The intrarater correlation coefficients were significant for the left, r(35) = .74, p < .05, and right, r(35) = .82, p < .05, amygdala, and the left, r(121) = .78, p < .05, and right, r(121) = .88 p < .05, hippocampi, respectively. A comparison of the AQ values for the amygdala and hippocampus between the two raters did not differ significantly. In addition, the coefficients of agreements for the left and right sides of the hippocampus and amygdala did not differ significantly.
Results
Population-Level Effects
A preliminary analysis showed no significant difference between cadaver and noncadaver brains; therefore, they were analyzed together. In addition, there was no significant difference between chimpanzees scanned on the portable MRI and those scanned at the MRI facility. For the amygdala, hippocampus, and total area, one-sample t tests were performed to evaluate whether the distributions in AQ scores differed significantly from zero, as would be predicted for a normally or bimodally distributed set of scores. Population-level right hemisphere asymmetries were found for the total region, t(59) = 2.28, p < .001, and hippocampus, t(59) = 3.63, p < .001. A borderline significant left hemisphere bias was found for the amygdala, t(59) = 1.86, p < .07.
Analysis of the categorical data largely confirmed the parametric results. Shown in Figure 1e are the average AQ values for the amygdala and hippocampus along with the standard errors for each structure. The distributions differed significantly from chance for the amygdala, χ2(2, N = 60) = 22.96, p < .01; hippocampus, χ2(2, N = 64) = 30.90, p < .001; and total, χ2(2, N = 54) = 9.10, p < .01. For the total, χ2(1, N = 45) = 6.42, p < .01, and hippocampus, χ2(1, N = 53) = 13.76, p < .01, the number of right hemisphere-dominant subjects was significantly higher than the number of left hemisphere-dominant subjects. No significant difference was found in the number of left and right hemisphere-dominant subjects for the amygdala.
Sex and Rearing Effects on Direction and Strength of Asymmetry
For this analysis, the AQ and ABS-AQ scores for each brain region served as dependent measures. Sex (male, female) and rearing history (wild-caught, mother-reared, human-reared) served as between-group variables in a factorial analysis of variance. In terms of the AQ scores, no significant main effects or interactions were found. For the ABS-AQ scores, there was a significant effect of sex on the total score F(1, 54) = 5.49, p < .01. Males (M = .110) were more lateralized than females (M = .058).
Intercorrelations Among Measures
The AQ scores for the amygdala and hippocampus did not significantly correlate, but the ABS-AQ scores for these two areas did significantly positively correlate, r(51) = .38, p < .01. Thus, subjects with larger hippocampus asymmetries similarly had larger asymmetries in the amygdala.
Amygdala and Hippocampus Volume
Shown in Table 1 are the mean volumes for the hippocampus, amygdala, and whole brain for the chimpanzee sample. A mixed model analysis of covariance was performed, with amygdala volume and hippocampus volume serving as the dependent measures. Sex and rearing history served as the between-group variables, and brain volume served as the covariate. No significant main effects or interactions were found. We also performed a partial correlation coefficient between age and the amygdala and hippocampus volumes, with brain volume serving as a covariate. No significant associations were found.
Table 1.
Mean (± SEM) Volumes of the Amygdala, Hippocampus, and Whole Brain
| Group | Amygdala | Hippocampus | Whole brain |
|---|---|---|---|
| Females | 582.08 ± 41.97 | 2,191.31 ± 84.28 | 361.05 ± 8.63 |
| Males | 628.51 ± 77.63 | 2,348.59 ± 127.10 | 401.11 ± 10.90 |
| Overall M | 602.81 ± 41.42 | 2,264.71 ± 74.47 | 379.74 ± 7.28 |
Note. Values for the amygdala and hippocampus are in cubic millimeters; whole brain values are in cubic centimeters.
Discussion
The results of this study are relatively straightforward. Chimpanzees showed a rightward asymmetry in the hippocampus, but no population-level bias for the amygdala. Rearing history of the subjects did not have a significant effect on the direction and strength of asymmetries or the overall volumes of the hippocampus and amygdala. Sex did have a significant effect on the strength of asymmetries, but not the direction or overall volumes of the hippocampus and amygdala.
The evidence of right-sided asymmetries in the hippocampus is consistent with the bulk of findings in human subjects (Agartz et al., 1999; Bigler et al., 1997; Bilir et al., 1998; Free et al., 1995; Hasboun et al., 1996; Pegues et al., 2003; Sullivan, Marsh, Mathalon, Lim, & Pfefferbaum, 1995; Watson et al., 1992). Similarly, the lack of population-level asymmetries in the amygdala for the chimpanzees is consistent with the general findings in human subjects based on meta-analyses (Mori et al., 1997; Soininen et al., 1994; Strakowski et al., 1999; Watson et al., 1992). Thus, our overall results in chimpanzees parallel the general findings of asymmetries in the limbic system of humans, which suggests homology in structural asymmetries at both the cortical and subcortical level.
Although several studies have looked at hippocampal and amygdalar volumes with regard to sex effects (Agartz et al., 1999; Bigler et al., 1997; Durston et al., 2001; Franklin et al., 2000; Gur et al., 2002; Szabo et al., 2001), only one, to our knowledge, has studied sex effects with regard to hippocampal and amygdalar asymmetries. The previous study examining sex differences in hippocampus and amygdalar asymmetries found that males have a larger hippocampal asymmetry in the right hemisphere compared to females, whereas our results showed that males are more lateralized than females, but the asymmetry is not directional (Narr et al., 2001). Both our study and Narr et al. found that neither males nor females have a larger amygdalar asymmetry compared to the other sex.
With respect to rearing effects, our findings are consistent with two previous studies in monkeys. In those studies, early life stress from either disrupted maternal care (Lyons et al., 2001) or human rather than conspecific rearing (Sanchez et al., 1998) was not found to correlate with hippocampal volumes. The previous studies in monkeys did not evaluate asymmetries in either the amygdala or hippocampus; thus, it is not clear whether species differences might be evident in terms of the effect of rearing on limbic system asymmetry.
As has been reported in studies of human MRI scans (Bilir et al., 1998; Brierley et al., 2002; Free et al., 1995; Gosche et al., 2001; Jack, Theodore, Cook, & McCarthy, 1995; Mayhew, 1992; Pruessner et al., 2000), delineating the amygdala and hippocampus from in vivo images was difficult in the ape brains because of variability in the structures used to define the regions and the lack of good resolution of these landmark areas using MRI. This is partially reflected in the lower interrater reliabilities reported here compared to measurements of other, more easily defined brain regions (e.g., Hopkins et al., 1998). One explanation for why an asymmetry was found for the hippocampus and not for the amygdala could be the lower interrater reliability for the amygdala. However, the results from the interrater correlation coefficients indicate that both raters found no asymmetry for the amygdala. It is possible that using a much longer scanning protocol could improve image quality, but, pragmatically, this presents some problems with apes because they are anesthetized for the procedure, and maintaining them in this state for longer periods of time increases the risk of injury.
In summary, these results suggest that the patterns seen in human volumetric MRI and asymmetry studies with the hippocampus and amygdala are, for the most part, also seen in the limbic system of chimpanzees. This includes a larger right compared to left hippocampal asymmetry, as well as no significant amygdalar asymmetry. These findings contribute to the increasing number of subcortical structures that have been found to show similar asymmetries in both humans and chimpanzees. Comparisons between cortical and subcortical structures that may be interconnected in the chimpanzee brain could be the focus of further studies using MRI technology to study comparative neuroanatomy. Moreover, functional correlates of asymmetries in the amygdala and hippocampus would be of comparative interest as a means of evaluating the evolution of the limbic system in relation to the behavior of primates, including humans.
Acknowledgments
This work was supported in part by National Institutes of Health Grants RR-00165, NS-42867, NS-36605, and HD-38051.
Contributor Information
Hani D. Freeman, Division of Psychobiology, Yerkes National Primate Research Center, Atlanta, Georgia
Claudio Cantalupo, Division of Psychobiology, Yerkes National Primate Research Center, and Language Research Center, Georgia State University.
William D. Hopkins, Division of Psychobiology, Yerkes National Primate Research Center, and Department of Psychology, Berry College
References
- Abe K. Modulation of the hippocampal long-term potentiation by the amygdala: A synaptic mechanism linking emotion and memory. Japanese Journal of Pharmacology. 2001;86:18–22. doi: 10.1254/jjp.86.18. [DOI] [PubMed] [Google Scholar]
- Agartz I, Momenan R, Rawlings RR, Kerich MJ, Hommer DW. Hippocampal volume in patients with alcohol dependence. Archives of General Psychiatry. 1999;56:356–363. doi: 10.1001/archpsyc.56.4.356. [DOI] [PubMed] [Google Scholar]
- Aggleton JP. The amygdala. Neurological aspects of emotion, memory, and mental dysfunction. Wiley-Liss; New York: 1992. [Google Scholar]
- Aggleton JP. The amygdala. A functional analysis. 2nd ed. Oxford University Press; New York: 2000. [Google Scholar]
- Bhatia S, Bookheimer SY, Gaillard WD, Theodore WH. Measurement of whole temporal lobe and hippocampus for MR volumetry: Normative data. Neurology. 1993;43:2006–2010. doi: 10.1212/wnl.43.10.2006. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Blatter DD, Anderson CV, Johnson SC, Gale SD, Hopkins RO, Burnett B. Hippocampal volume in normal aging and traumatic brain injury. American Journal of Neuroradiology. 1997;18:11–23. [PMC free article] [PubMed] [Google Scholar]
- Bilir E, Craven W, Hugg J, Gilliam F, Martin R, Faught E, Kuzniecky R. Volumetric MRI of the limbic system: Anatomic determinants. Neuroradiology. 1998;40:138–144. doi: 10.1007/s002340050554. [DOI] [PubMed] [Google Scholar]
- Bradshaw J, Rogers LJ. The evolution of lateral asymmetries, language, tool use and intellect. Academic Press; San Diego, CA: 1993. [Google Scholar]
- Brierley B, Shaw P, David AS. The human amygdala: A systematic review and meta-analysis of volumetric magnetic resonance imaging. Brain Research Reviews. 2002;39:84–105. doi: 10.1016/s0165-0173(02)00160-1. [DOI] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. Mechanisms of emotional arousal and lasting declarative memory. Trends in Neurosciences. 1998;21:294–299. doi: 10.1016/s0166-2236(97)01214-9. [DOI] [PubMed] [Google Scholar]
- Cantalupo C, Freeman HD, Hopkins WD. Patterns of cerebellar asymmetries in great apes as revealed by MRI. Brain, Behavior and Evolution. in press. [Google Scholar]
- Cantalupo C, Hopkins WD. Evolution of Broca's area: Asymmetry of the inferior frontal cortex (Brodmann's area 44) in great apes as revealed by MRI. Nature. 2001 November 29;414:505. doi: 10.1038/35107134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantalupo C, Pilcher DL, Hopkins WD. Are planum temporale and sylvian fissure asymmetries directly correlated?: A MRI study in great apes. Neuropsychologia. 2003;41:1975–1981. doi: 10.1016/s0028-3932(02)00288-9. [DOI] [PubMed] [Google Scholar]
- Corballis MC. The lopsided brain: Evolution of the generative mind. Oxford University Press; New York: 1992. [Google Scholar]
- Crow TJ. Sexual selection, timing and the descent of man. A theory on the genetic origins of language. Current Psychology of Cognition. 1998;17:1079–1114. [Google Scholar]
- De Bellis MD, Casey BJ, Dahl RE, Birmaher B, Williamson DE, Thomas KM, et al. A pilot study of amygdala volumes in pediatric generalized anxiety disorder. Biological Psychiatry. 2000;48:51–57. doi: 10.1016/s0006-3223(00)00835-0. [DOI] [PubMed] [Google Scholar]
- Denenberg VH. Laterality in animals: Brain and behavioral asymmetries and the role of early experiences. In: Molfese DL, Segalowitz SJ, editors. Brain lateralization in children: Developmental implications. Guilford; New York: 1988. pp. 59–72. [Google Scholar]
- Durston S, Hulshoff Pol HE, Hilleke E, Casey BJ, Giedd JN, Buitelaar JK, Van Engeland H. Anatomical MRI of the developing human brain: What have we learned? Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:1012–1020. doi: 10.1097/00004583-200109000-00009. [DOI] [PubMed] [Google Scholar]
- Fernandez-Carriba S, Loeches A, Morcillo A, Hopkins WD. Asymmetry of facial expression of emotions by chimpanzees. Neuropsychologia. 2002;40:1523–1533. doi: 10.1016/s0028-3932(02)00028-3. [DOI] [PubMed] [Google Scholar]
- Franklin MS, Kraemer GW, Shelton SE, Baker E, Kalin NH, Uno H. Gender differences in brain volume and size of corpus collosum and amygdala of rhesus monkey measured from MRI images. Brain Research. 2000;852:263–267. doi: 10.1016/s0006-8993(99)02093-4. [DOI] [PubMed] [Google Scholar]
- Free SL, Bergin PS, Fish DR, Cook MJ, Shorvon SD, Stevens JM. Methods for normalization of hippocampal volumes measured with MR. American Journal of Neuroradiology. 1995;16:637–643. [PMC free article] [PubMed] [Google Scholar]
- Gannon PJ, Holloway RL, Broadfield DC, Braun AR. Asymmetry of chimpanzee planum temporale: Humanlike pattern of Wernicke's brain language area homologue. Science. 1998 January 9;279:220–222. doi: 10.1126/science.279.5348.220. [DOI] [PubMed] [Google Scholar]
- Gilissen E. Structural symmetries and asymmetries in human and chimpanzee brains. In: Falk D, Gibson KR, editors. Evolutionary anatomy of the primate cerebral cortex. Cambridge University Press; Cambridge, England: 2001. pp. 187–215. [Google Scholar]
- Gosche KM, Mortimer JA, Smith CA, Markesbery WA, Snowdon D. An automated technique for measuring hippocampal volumes from MR imaging studies. American Journal of Neuroradiology. 2001;22:1686–1689. [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Gunning-Dixon F, Bilker WB, Gur RE. Sex differences in temporo-limbic and frontal brain volumes of healthy adults. Cerebral Cortex. 2002;12:998–1003. doi: 10.1093/cercor/12.9.998. [DOI] [PubMed] [Google Scholar]
- Hasboun D, Chantome M, Zouaoui A, Sahel M, Deladoeuille M, Sourour N, et al. MR determination of hippocampal volume: Comparison of three methods. American Journal of Neuroradiology. 1996;17:1091–1098. [PMC free article] [PubMed] [Google Scholar]
- Hauser MC. Right hemisphere dominance in the production of facial expression in monkeys. Science. 1993 July 23;261:475–477. doi: 10.1126/science.8332914. [DOI] [PubMed] [Google Scholar]
- Hellige J. Hemispheric asymmetry. Harvard University Press; Cambridge, MA: 1993. [Google Scholar]
- Holloway RL, De La Coste-Lareymondie MC. Brain endocast asymmetry in pongids and hominids: Some preliminary finding on the paleontology of cerebral dominance. American Journal of Physical Anthropology. 1982;58:101–110. doi: 10.1002/ajpa.1330580111. [DOI] [PubMed] [Google Scholar]
- Hook-Costigan MA, Rogers LJ. Lateralized use of the mouth in production of vocalizations by marmosets. Neuropsychologia. 1998;36:1265–1273. doi: 10.1016/s0028-3932(98)00037-2. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Marino L. Asymmetries in cerebral width in nonhuman primate brains as revealed by magnetic resonance imaging (MRI) Neuropsychologia. 2000;38:493–499. doi: 10.1016/s0028-3932(99)00090-1. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Marino L, Rilling JK, MacGregor LA. Planum temporale asymmetries in great apes as revealed by magnetic resonance imaging (MRI) NeuroReport. 1998;9:2913–2918. doi: 10.1097/00001756-199808240-00043. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Morris RD. Laterality for visual spatial processing in two language-trained chimpanzees (Pan troglodytes) Behavioral Neuroscience. 1989;103:227–234. doi: 10.1037//0735-7044.103.2.227. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Pilcher DL. Neuroanatomical localization of the motor hand area with magnetic resonance imaging: The left hemisphere is larger in great apes. Behavioral Neuroscience. 2001;115:1159–1164. [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Pilcher DL, MacGregor L. Sylvian fissure asymmetries in nonhuman primates revisited. A comparative MRI study of the relationship between neuroanatomical asymmetry and interhemispheric connectivity in primates: Implication for the evolution of functional asymmetries. Behavioral Neuroscience. 2000;114:739–748. doi: 10.1037//0735-7044.114.4.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Washburn DA. Do right- and left-handed monkeys differ on cognitive measures? Behavioral Neuroscience. 1994;108:1207–1212. doi: 10.1037//0735-7044.108.6.1207. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr., Theodore WH, Cook M, McCarthy G. MRI-based hippocampal volumetrics: Data acquisition, normal ranges, and optimal protocol. Magnetic Resonance Imaging. 1995;13:1057–1064. doi: 10.1016/0730-725x(95)02013-j. [DOI] [PubMed] [Google Scholar]
- Jason GW, Cowey A, Weiskratz L. Hemispheric asymmetry for a visual-spatial task in monkeys. Neuropsychologia. 1984;22:777–784. doi: 10.1016/0028-3932(84)90102-7. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotional memory systems in the brain. Behavioural Brain Research. 1993;58:69–79. doi: 10.1016/0166-4328(93)90091-4. [DOI] [PubMed] [Google Scholar]
- LeMay M. Morphological cerebral asymmetries of modern man, fossil man and nonhuman primates. In: Harnad SR, Steklis HD, Lancaster J, editors. Annals of the New York Academy of Sciences: Vol. 280. Origin and evolution of language and speech. New York Academy of Sciences; New York: 1976. pp. 349–366. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Yang C, Sawyer-Glover AM, Moseley ME, Schatzberg AF. Early life stress and inherited variation in monkey hippocampal volumes. Archives of General Psychiatry. 2001;58:1145–1151. doi: 10.1001/archpsyc.58.12.1145. [DOI] [PubMed] [Google Scholar]
- Massana G, Serra-Grabulosa JM, Salgado-Pineda P, Gastó C, Junqué C, Massana J, et al. Amygdalar atrophy in panic disorder patients detected by volumetric magnetic resonance imaging. NeuroImage. 2003;19:80–90. doi: 10.1016/s1053-8119(03)00036-3. [DOI] [PubMed] [Google Scholar]
- Mayhew TM. A review of recent advances in sterology for quantifying neural structure. Journal of Neurocytology. 1992;21:313–328. doi: 10.1007/BF01191700. [DOI] [PubMed] [Google Scholar]
- Mori E, Yoneda Y, Yamashita H, Hirono N, Ikeda M, Yamadori A. Medial temporal structures relate to memory impairment in Alzheimer's disease: An MRI volumetric study. Journal of Neurology Neurosurgery Psychiatry. 1997;63:214–221. doi: 10.1136/jnnp.63.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narr KL, Thompson PM, Sharma T, Moussai J, Blanton R, Anvar B, et al. Three-dimensional mapping of temporo-limbic regions and the lateral ventricles in schizophrenia: Gender effects. Biological Psychiatry. 2001;50:84–97. doi: 10.1016/s0006-3223(00)01120-3. [DOI] [PubMed] [Google Scholar]
- Pegues MP, Rogers LJ, Amend D, Vinogradov S, Deicken RF. Anterior hippocampal volume reduction in male patients with schizophrenia. Schizophrenia Research. 2003;60:105–115. doi: 10.1016/s0920-9964(02)00288-8. [DOI] [PubMed] [Google Scholar]
- Pilcher DL, Hammock EAD, Hopkins WD. Cerebral volumetric asymmetries in non-human primates: A magnetic resonance imaging study. Laterality. 2001;6:165–179. doi: 10.1080/13576500042000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Li LM, Serles W, Pruessner M, Collins DL, Kabani N, et al. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: Minimizing the discrepancies between laboratories. Cerebral Cortex. 2000;10:433–442. doi: 10.1093/cercor/10.4.433. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Uecker A, Caselli RJ, Lewis S, Bandy D, de Leon MJ, et al. Hippocampal volumes in cognitively normal persons at genetic risk for Alzheimer's disease. Annals of Neurology. 1998;2:288–291. doi: 10.1002/ana.410440226. [DOI] [PubMed] [Google Scholar]
- Rogers LJ, Andrew RJ. Comparative vertebrate lateralization. Oxford University Press; Oxford, England: 2002. [Google Scholar]
- Sanchez MM, Hearn EF, Do D, Rilling JK, Herndon JG. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Research. 1998;812:38–49. doi: 10.1016/s0006-8993(98)00857-9. [DOI] [PubMed] [Google Scholar]
- Scahill RI, Frost C, Jenkins R, Whitwell J, Rossor M, Fox N. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Archives of Neurology. 2003;60:989–994. doi: 10.1001/archneur.60.7.989. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. Journal of Neuroscience. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder PJ, Bilder RM, Wu H, Bogerts B, Lieberman JA. Cerebellar volume asymmetries are related to handedness: A quantitative MRI study. Neuropsychologia. 1995;33:407–419. doi: 10.1016/0028-3932(94)00125-9. [DOI] [PubMed] [Google Scholar]
- Soininen HS, Partanen K, Pitkanen A, Vainio P, Hanninen T, Hallikainen Koivisto K, Riekkinen PJ., Sr. Volumetric MRI analysis of the amygdala and the hippocampus in subjects with age-associated memory impairment: Correlation to visual and verbal memory. Neurology. 1994;44:1660–1668. doi: 10.1212/wnl.44.9.1660. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, DelBello MP, Sax KW, Zimmerman ME, Shear PK, Hawkins JM, Larson ER. Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Archives of General Psychiatry. 1999;56:254–260. doi: 10.1001/archpsyc.56.3.254. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Age-related decline in MRI volumes of temporal lobe gray matter but not hippocampus. Neurobiology of Aging. 1995;16:591–606. doi: 10.1016/0197-4580(95)00074-o. [DOI] [PubMed] [Google Scholar]
- Szabo CA, Xiong J, Lancaster JL, Rainey L, Fox P. Amygdalar and hippocampal volumetry in control participants: Differences regarding handedness. American Journal of Neuroradiology. 2001;22:1342–1345. [PMC free article] [PubMed] [Google Scholar]
- Toga AW, Thompson M. Mapping brain asymmetry. Nature Reviews Neuroscience. 2003;4:37–48. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- Watson C, Andermann F, Gloor P, Jones-Glotman M, Peters T, Evans A, et al. Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurology. 1992;42:1743–1750. doi: 10.1212/wnl.42.9.1743. [DOI] [PubMed] [Google Scholar]
- Yeni-Komshian GH, Benson DA. Anatomical study of cerebral asymmetry in the temporal lobe of humans, chimpanzees, and rhesus monkeys. Science. 1976 April 23;192:387–389. doi: 10.1126/science.816005. [DOI] [PubMed] [Google Scholar]