Abstract
The effect of birth order on hand preference was assessed in a sample of 154 captive-born chimpanzees. Subjects were classified as first, middle, or latter born using 2 classification criteria based on their birth order. Hand preference was measured using a task that elicited coordinated bimanual actions. Significant birth-order effects were found for both classification criteria, with first- and latter-born subjects exhibiting a lesser degree of right-handedness compared with middle-born subjects. These data suggest that biological rather than sociological factors play a greater role in explaining the observed birth-order effects on hand preference in humans.
Approximately 85% to 90% of humans report themselves as being right-handed (Annett, 1985; Porac & Coren, 1981). Explaining the pervasive degree of right-handedness in the human species has fostered considerable theoretical debate and empirical investigation. Genetic, biological, and environmental models have been proposed to explain this population-level right-handedness, each with varying amounts of empirical support (Annett, 1999; Collins, 1985; Corballis, 1997; Laland, Kumm, Van Horn, & Feldman, 1995; Previc, 1991; Provins, 1997; Trevarthen, 1996). Equally important are theoretical models proposed to explain the occurrences of left-handedness or non-right-handedness in human populations. That genetic factors may play a role is supported by the observation that approximately 70% of individuals born to two left-handed parents are right-handed, which is significantly lower than the proportion of fight-handed individuals born to two right-handed parents (McGee & Cozad, 1980; McManus & Bryden, 1992). Genetic explanations account for only part of the variance, however, leaving error or other factors to explain the remaining variability. Rather than emphasize genetic factors, others have suggested that both pre- and postnatal environmental factors play a role in determining non-right-handedness. Such factors include but are not limited to birth season, birth order, maternal age, and prenatal hormones. In these models, right-handedness is perceived as being the normative path of development, and therefore non-right-handedness reflects a deviation from normal development that is due to pathological factors (see Satz, Soper, & Orsini, 1988). Of specific interest to this study are the reports focusing on the effect of birth order on hand preference in humans.
Bakan and colleagues (Bakan, 1971; Bakan, Dibb, & Reed, 1973) first reported and theorized about the relationship between birth order and hand preference in humans. Bakan (1971) reported that there was a higher percentage of left-handedness in first- and latter-born offspring (defined as more than 4 birth orders) and postulated that the effects were due to birth trauma experienced by offspring born to primiparous or older women. Bakan (1971) proposed that birth trauma is more likely in women who are giving birth for the first time or for older women who present a higher risk group for prenatal insult. Researchers who have attempted to replicate Bakan's early reports have come up with equivocal results, with most of the discrepancies between studies centering on the issue of sampling procedures (Annett & Ockwell, 1980; Badian, 1983; Bakan, 1977, 1978; Coren & Porac, 1980; Dellatolas, Curt, & Lellouch, 1991; Hicks, Evans, & Pellegrini, 1978; McKeever, Suter, & Rich, 1995; Nachshon & Denno, 1986; Perelle & Ehrman, 1994; Schwartz, 1977; Searleman, Porac, & Coren, 1989; Tan & Nettleton, 1980). Specifically, there are significant relationships between socioeconomic status (SES), rate of pregnancy, and birth complications that are likely due to factors associated with maternal lifestyle (such as nutritional habits, medical care, etc.). For example, less well-off individuals typically have more offspring, poorer prenatal care, poorer nutritional habits, and more birth complications compared with well-off individuals (Birch & Gussow, 1970). According to Bakan (1977), it is critical to have equal representation of subjects from various SES backgrounds to control for these extraneous variables, which he argues has not been the case in studies in which his findings were not replicated (e.g., Hubbard, 1971; Schwartz, 1977).
The purpose of this study was to examine the effect of birth order on hand preference in a sample of captive chimpanzees. Theoretically, the effect of birth order on hand preference in captive chimpanzees (as well as other primates) is of interest because the hypothesized SES confounding variables debated in the literature on humans would not be potential confounds in a sample of captive chimpanzees (or other primates) where the environment and access to resources are more uniform. In other words, the methodological issue of whether there was adequate sampling of subjects to control for SES variables would never be considered in a sample of nonhuman primates. Presumably, any significant effect of birth order on hand preference would be solely due to biological factors associated with parity or maternal age or both. Pragmatically, chimpanzees offer a good model for testing the effect of birth order on hand preference because they are biologically and genetically very similar to humans, but there is no socioeconomic variation in a captive colony. Additionally, chimpanzees have been reported to exhibit population-level right-handedness, and therefore non-right-handedness can be viewed as reflecting deviation from a species-typical norm (Hopkins, 1996; but see McGrew & Marchant, 1997). Finally, chimpanzees have a relatively long gestation period (approximately 230 days), exhibit stages of prenatal development comparable to humans, display periparturitional behaviors that resemble many of those observed in humans (Nissen & Yerkes, 1943), and exhibit a wide range of similar puerperal pathologies (Dahl, 1999).
Method
Subjects
There were 154 subjects ranging in age from 3 to 57 years (M = 16.12, SD = 11.26), all of whom were housed at the Yerkes Regional Primate Research Center (YRPRC). Of the 154 subjects, there were 86 females and 68 males, respectively. Of the 86 females, 31 were reared by their mothers, and 55 were reared in the nursery. Of the 68 males, 22 were reared by their mothers, and 46 were reared in the nursery. Mother-reared chimpanzees were those reared by their biological, conspecific mother for more than 30 days of life. Nursery-reared subjects were those that were brought to the YRPRC nursery before they were 31 days old. The standard protocol for hand rearing chimpanzees has been described in detail elsewhere (Bard, 1996).
Procedure
Hand preference was assessed using a task designed to elicit coordinated bimanual actions, referred to as the tube task. The procedure for this task has been described in detail elsewhere (Hopkins, 1995). Briefly, peanut butter is smeared on the inside edge of polyvinyl chloride (PVC) tubes approximately 15.0 cm in length and 2.5 cm in diameter. Peanut butter is smeared on beth ends of the PVC pipe and is placed far enough down the tube such that the chimpanzees cannot lick the contents completely off with their mouths but rather must use their fingers to remove the substrate. The PVC tubes are handed to the subjects in their home cages, and a focal sampling is used to collect individual data from each subject. The hand and finger used to extract the peanut butter were recorded as either right or left by the experimenter. Data were collected until the subjects either dropped the tube, stopped extracting peanut butter for a period of 10 s, or returned the PVC pipe back to the experimenter. Each time the subjects extracted food from the PVC pipe, the hand used was recorded. No attempt was made to code for bouts of responses rather than the individual motor events. Some have argued that this is critical in evaluating individual hand preferences because there is a lack of independence of data points (McGrew & Marehant, 1997), but there is no empirical evidence to support this contention (Hopkins, 1999). Each subject received two test sessions separated by at least 1 day, and a minimum of 25 responses were obtained from each subject. A test–retest correlation indicated reliable hand use between test sessions (r = .66, p < 01). A handedness index (HI) was derived to reflect the magnitude and direction of hand preference for the tube task. The HI score was determined by subtracting the number of left-from the number of right-hand responses and dividing by the total number of responses: (R − L)/(R + L). Positive values reflected right-hand biases and negative values reflected left-hand biases. The absolute value of the HI score reflected the magnitude of hand preference.
In addition to the measures of hand preference, the birth order of the subject and the ages of the mothers at the time of the subjects' births were recorded for each subject. Birth order and maternal age data were available from the animal records of the YRPRC. From these records, it was determined that the average parity of the YRPRC chimpanzees was 4.35 individuals (live + still births only) with a standard deviation of 2.37. Based on this calculation, subjects with parities of six or higher (referred to as the sixth+ group) were considered latter-born subjects. These subjects were compared with the subjects comprising the remaining five birth orders, including first, second, third, fourth, and fifth, respectively. Based on this classification scheme, the sample sizes for each birth-order group were 25, 28, 24, 18, 15, and 44 individuals.
Results
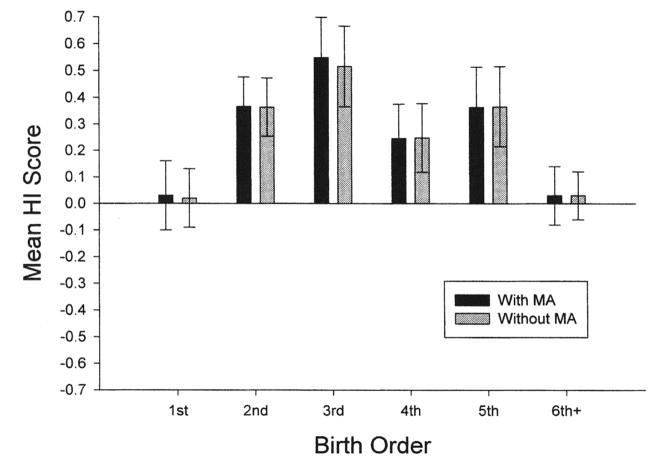
In a preliminary analysis, it was found that maternal age and literal birth order were significantly positively correlated, r(152) = ,78, p < .001. Thus, two analyses of variance (ANOVAs) were performed with the HI score serving as the dependent variable. Sex, rearing history of the subject, and birth order were the between group variables. In the first analysis, maternal age served as a covariate, whereas in the second analysis, maternal age was not considered as a covariate. This was done to determine whether maternal age altered the effects of birth order on hand preference in a significant way. The mean HI scores for each birth order group with and without maternal age in the statistical model can be seen in Figure 1. A significant main effect for birth order, F(5, 124) = 2.83, p < .02, and sex, F(1, 122) = 5.46, p < .03, was found with maternal age serving as a covariate. Males (M = 0.386) had significantly higher HI scores than females (M = 0.143). With respect to the birth-order variable, Tukey's honestly significant difference post hoc tests indicated that the mean HI for the second, third, and fourth birth orders differed significantly from the first and sixth+ birth orders. No other significant differences were found. For the ANOVA that did not include maternal age as a covariate, the same results were found with significant main effects for birth order, F(5, 128) = 2.72, p < .02, and sex, F(1, 128) = 6.17, p < .02.
Figure 1.
Mean handedness index (HI) scores (+SE) for the first-, second-, third-, fourth-, fifth-, and sixth+-born chimpanzees with maternal age (MA) or without MA in the statistical model.
It should be emphasized that rearing condition is a highly relevant extraneous variable in this study because females that do not keep their offspring are likely to produce more offspring than those that do. The removal of the offspring (because of either poor maternal behavior or experimental purposes) would result in a cessation in lactation and subsequent recurrence of sexual cyclicity and receptivity. None of the analyses revealed significant main effects or interactions between birth order and the rearing history of the subject. The mean HI scores for subjects from each birth order and rearing condition are shown in Table 1. As can be seen, the general pattern was similar for each cohort of subjects reared by humans or by chimpanzees. Thus, the significant effect of birth order on hand preference cannot be due to increased incidence of nursery-reared subjects, particularly in the sixth+ group.
Table 1.
Mean (±SE) Handedness Index Scores for Each Birth-Order Group and Rearing Condition
| Birth order |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st |
2nd |
3rd |
4th |
5th |
6th+ |
|||||||
| Rearing condition | M | SE | M | SE | M | SE | M | SE | M | SE | M | SE |
| Mother reared | 0.125 | 0.17 | 0.380 | 0.17 | 0.592 | 0.27 | 0.130 | 0.20 | 0.449 | 0.24 | −0.071 | 0.16 |
| Nursery reared | −0.078 | 0.14 | 0.347 | 0.13 | 0.440 | 0.15 | 0.366 | 0.16 | 0.282 | 0.19 | 0.131 | 0.10 |
Rather than use the HI scores, we examined the relationship between birth order and hand preference using a categorical measure of handedness. Specifically, chimpanzees with negative HI scores or an HI score of 0 were classified as non-right-handed. Chimpanzees with a positive HI score were classified as right-handed. We then compared the frequency of right- and non-right-handed subjects in relation to birth order using a 5 × 2 chi-square test of independence. This analysis revealed a significant interaction between birth order and hand preference: χ2(5, N = 154) = 14.52, p < .02. The dislribution of right- and non-right-handed subjects can be seen in Table 2. As can be seen, there are more right- than non-right-handed subjects in the second, third, fourth, and fifth birth-order groups compared with the first and sixth+ birth-order groups. These results largely confirm the findings that resulted from ANOVAs.
Table 2.
Distribution of Right- and Non-Right-Handed Subjects in Relation to Birth Order
| Birth order | Number of non-right-handed |
Number of right-handed |
Percentage of right-handed |
|---|---|---|---|
| 1st | 12 | 13 | 52 |
| 2nd | 7 | 21 | 75 |
| 3rd | 4 | 20 | 83 |
| 4th | 5 | 13 | 72 |
| 5th | 4 | 11 | 73 |
| 6th | 23 | 21 | 48 |
Discussion
The results of this study indicate that birth order has a significant effect on hand preference in captive chimpanzees. This conclusion is supported by the results derived from both the ANOVAs and chi-square analyses. Moreover, these effects were evident even after statistically controlling for maternal age. Taken together, these results strongly support a biological interpretation of the effect of birth order on hand preference in humans, although these data do not speak directly to the mechanism that results in a higher degree of non-fight-handedness in first- and latter-born subjects. It is important to emphasize that birth order per se is probably not the critical variable in determining hand preference, but rather birth order is associated with risk factors that correspond to increasing parity and maternal age.
Although the results do not speak to the mechanism that results in reduced right-handedness in first- and latter-born chimpanzees, several possible explanations seem warranted. First, Bakan's (1977) hypothesis that the first- and latter-born offspring are subject to birth trauma is clearly a possibility. Although there are higher incidences of puerperal pathology in older females (Dahl, 1999), it is not clear whether primiparous or older multi-parous female chimpanzees exhibit lesser or greater degrees of birth trauma or even exhibit longer or shorter periods of labor. Nissen and Yerkes (1943) reported that primiparous females had more prolonged labor than multiparous females, but they presented only descriptive information and no quantitative data. Neonatal outcomes such as parity and maternal age have been reported to positively correlate with birth weight in monkeys and chimpanzees (Nissen & Yerkes, 1943; Silk, Short, Roberts, & Kushitz, 1993). In this sample, neonatal birth weights and gestation lengths were available from the animal records in 77 subjects. Birth order positively correlated with birth weight, r(75) = .336, p < .007, but not gestation length, r(75) = .054, ns. Maternal age did not significantly correlate with either gestation length, r(75) = −.081, ns, or birth weight, r(75) = .136, ns. Taken together, these data suggest that early born chimpanzees have lower birth weights, which may be interpreted as indicators of prenatal birth stress or prematurity, factors that are known to correlate with hand preference in humans (Petridou et al., 1994; Searleman et al., 1989).
A second explanation could be variations in the prenatal environment of primiparous and older multiparous females. Some have suggested that prenatal sex hormones such as estrogen (Hines, 1982) and testosterone (Geschwind & Galaburda, 1985) can differentially affect the development of each cerebral hemisphere (see Wisniewski, 1998, for a review). Previous studies in chimpanzees revealed that higher than normal levels of anogenital swelling occur in first pregnancies (Wallis & Goodall, 1993). More recent data indicate that swelling during pregnancy is caused by fluctuations in the estrogens and progesterone (Dahl, 1999; Dahl & Hopkins, 1999) and that the amount of swelling varies with parity and outcome. An extended and exaggerated swelling pattern occurs in many low-parity pregnancies (consistent with the findings of Wallis & Goodall, 1993) which, in light of previous studies on steroid control of anogenital swelling, is probably caused by a sensitivity of the mother to relatively low estrogen concentrations. Moreover, significantly reduced swelling occurs in pregnancies leading to puerperal pathology, such as stillbirth, a finding also consistent with the likelihood of low levels of estrogens (Dahl, 1999), and such a reduced swelling occurs with high incidence at high parity. It follows that a pathological steroid environment in utero may be contributing to the proximate causation of non-right-handedness.
Finally, both a birth trauma and hormonal explanation together could explain the higher incidence of non-right-handedness. Non-right-handedness in first-born individuals could be due to factors related to birth trauma or periparturitional stress, whereas non-right-handedness in latter-born subjects could be due to hormonal factors related to increasing maternal age. Clearly these different explanations warrant further investigation in both human and nonhuman species.
The effects reported here are for the tube task. Hopkins and Pearson (2000) previously reported that hand preferences for the tube task do not correlate with other measures of hand preference, including quadrupedal and bipedal reaching, simple reaching, or bimanual feeding, but they do correlate with a second measure requiring coordinated bimanual actions. Thus, it could be argued that the effects of birth order on hand preference are constrained by the type of task used to assess hand preference. To some extent there is support for this argument. For example, a comparison of HI values for a measure of bimanual feeding (see Hopkins, 1994, for a description) does not reveal a significant effect for birth order. However, when a cumulative handedness score derived from two to six separate measures of hand preference is used as the dependent measure, a pattern of findings is observed that is comparable to those reported for the tube task (see Figure 2). Although the degree of right-handedness is higher in the first- and latter-born condition relative to the tube task alone, the effect of birth order is significant for this measure. Suffice to say, it appears that certain motor tasks are more sensitive than others to birth-order effects in chimpanzees, and with additional testing, this may prove to be the case in human subjects.
Figure 2.
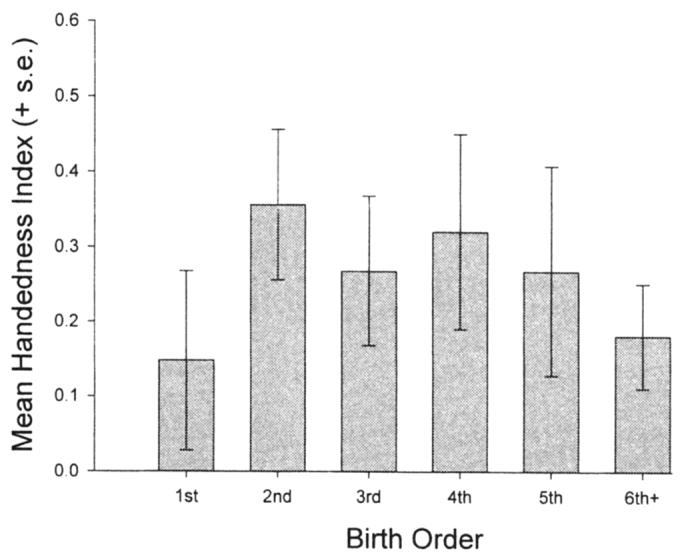
Mean handedness index scores (+SE) for the first-, second-, third-, fourth-, fifth-, and sixth+-born chimpanzees for a cumulative handedness score derived from multiple measures of hand preference (see Hopkins & Pearson, 2000).
In conclusion, birth order affects the development of hand preference for coordinated bimanual actions in chimpanzees, a finding that supports biological explanations for the expression of birth-order effects on handedness reported in human subjects. It should be emphasized that these effects were evident in both mother- and nursery-reared chimpanzees, thus removing the confound of rearing history as a potential explanation for these findings. Taken together, these data suggest that risk factors associated with maternal experience (parity) or age or both that may be marked by higher incidences of non-right-handedness are also manifest in chimpanzees. In short, non-right-handedness may serve as a marker of a pathological or atypical pregnancy or perinatal event. The extent to which non-right-handedness reflects atypical lateralization of the nervous system will require further examination. What is clear from this study is that sociological factors, such as poorer diet or prenatal care, do not explain these findings. It is from this perspective that chimpanzees offer an excellent species for studies focusing on the potential influence of biological factors on the development of lateralization of function in primates, including humans.
Acknowledgments
This research was supported by National Institutes of Health Grants NS-29574, NS-36605, and RR-00165 to the Yerkes Regional Primate Research Center. We are especially thankful to Sue Setzkorn and her staff for assistance in obtaining birth and health records on the chimpanzees. The Yerkes Regional Primate Research Center is fully accredited by the American Association for Accreditation of Laboratory Animal Care. The American Psychological Association's guidelines for the ethical treatment of animals were adhered to during all aspects of this study.
Contributor Information
William D. Hopkins, Department of Psychology, Berry College and Division of Psychobiology, Yerkes Regional Primate Research Center, Emory University
Jeremy F. Dahl, Division of Psychobiology, Yerkes Regional Primate Research Center, Emory University.
References
- Annett M. Left, right, hand, and brain: The right-shift theory. Erlbaum; Hillsdale, NJ: 1985. [Google Scholar]
- Annett M. Handedness and cerebral dominance: The right shift theory. Journal of Neuropsychiatry. 1999;10:459–469. doi: 10.1176/jnp.10.4.459. [DOI] [PubMed] [Google Scholar]
- Annett M, Ockwell A. Birth order, birth stress and handedness. Cortex. 1980;16:181–187. doi: 10.1016/s0010-9452(80)80033-5. [DOI] [PubMed] [Google Scholar]
- Badian NA. Birth order, maternal age, season of birth, and handedness. Cortex. 1983;19:451–463. doi: 10.1016/s0010-9452(83)80027-6. [DOI] [PubMed] [Google Scholar]
- Bakan P. Handedness and birth order. Nature. 1971;195:229. doi: 10.1038/229195a0. [DOI] [PubMed] [Google Scholar]
- Bakan P. Left handedness and birth order revisited. Neuropsychologia. 1977;15:837–839. doi: 10.1016/0028-3932(77)90018-5. [DOI] [PubMed] [Google Scholar]
- Bakan P. Handedness and birth order: A critical note on a critical note. Perceptual and Motor Skill. 1978;46:556. doi: 10.2466/pms.1978.46.2.556. [DOI] [PubMed] [Google Scholar]
- Bakan P, Dibb G, Reed P. Handedness and birth stress. Neuropsychologia. 1973;11:363–366. doi: 10.1016/0028-3932(73)90050-x. [DOI] [PubMed] [Google Scholar]
- Bard KA. Responsive care: Behavioral intervention for nursery-reared chimpanzees. Jane Goodall Institute; P.O. Box 14890, Silver Spring, MD: 1996. pp. 20911–4890. Available from the. [Google Scholar]
- Birch HD, Gussow JD. Disadvantaged children: Health, nutrition and school failure. Harcourt, Brace, & Word; New York: 1970. [Google Scholar]
- Collins RL. On the inheritance of direction and degree of asymmetry. In: Glick S, editor. Cerebral lateralization in nonhuman species. Academic Press; Orlando, FL: 1985. pp. 123–135. [Google Scholar]
- Corballis MC. The genetics and evolution of handedness. Psychological Review. 1997;104:714–727. doi: 10.1037/0033-295x.104.4.714. [DOI] [PubMed] [Google Scholar]
- Coren S, Porac C. Birth factors and laterality: Effects of birth order, paternal age, and birth stress on four indices of lateral preference. Behavior Genetics. 1980;10:123–138. doi: 10.1007/BF01066263. [DOI] [PubMed] [Google Scholar]
- Dahl JF. Perineal swelling during pregnancy in common chimpanzees and puerperal pathology. Journal of Medical Primatology. 1999;28:129–141. doi: 10.1111/j.1600-0684.1999.tb00260.x. [DOI] [PubMed] [Google Scholar]
- Dahl JF, Hopkins WD. Perineal swelling during pregnancy in chimpanzees (Pan troglodytes) is an external marker of steroid hormones. American Journal of Primatology. 1999;49:47–48. [Google Scholar]
- Dellatolas G, Curt F, Lellouch J. Birth order and month of birth are not related with handedness in a sample of 9370 young men. Cortex. 1991;27:137–140. doi: 10.1016/s0010-9452(13)80277-8. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Galaburda AM. Cerebral lateralization. Biological mechanisms, associations, and pathology: I. A hypothesis and a program for research. Archives of Neurology. 1985;42:428–459. doi: 10.1001/archneur.1985.04060050026008. [DOI] [PubMed] [Google Scholar]
- Hicks RA, Evans EA, Pellegrini RJ. Correlation between handedness and birth order: Compilation of five studies. Perceptual and Motor Skills. 1978;46:53–54. doi: 10.2466/pms.1978.46.1.53. [DOI] [PubMed] [Google Scholar]
- Hines M. Prenatal hormones and sex differences in human behavior. Psychological Bulletin. 1982;92:56–80. [PubMed] [Google Scholar]
- Hopkins WD. Hand preferences for bimanual feeding in 140 captive chimpanzees (Pan troglodytes): Rearing and ontogenetic factors. Developmental Psychobiology. 1994;27:395–407. doi: 10.1002/dev.420270607. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Hand preferences for a coordinated bimanual task in 110 chimpanzees (Pan troglodytes): Cross-sectional analysis. Journal of Comparative Psychology. 1995;109:291–297. doi: 10.1037/0735-7036.109.3.291. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Chimpanzee handedness revisited: 55 years since Finch (1941) Psychonomic Bulletin and Review. 1996;3:449–457. doi: 10.3758/BF03214548. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. On the other hand: Statistical issues in the assessment and interpretation of hand preference data in nonhuman primates. International Journal of Primatology. 1999;20:851–866. [Google Scholar]
- Hopkins WD, Pearson K. Chimpanzee (Pan troglodytes) handedness: Variability across multiple measures of hand use. Journal of Comparative Psychology. 2000;114:126–135. doi: 10.1037/0735-7036.114.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard JI. Handedness is not a function of birth order. Nature. 1971;232:276–277. doi: 10.1038/232276a0. [DOI] [PubMed] [Google Scholar]
- Laland KN, Kumm J, Van Horn JD, Feldman MW. A gene-cuiture model of human handedness. Behavior Genetics. 1995;25:433–445. doi: 10.1007/BF02253372. [DOI] [PubMed] [Google Scholar]
- McGee MG, Cozad T. Population genetic analysis of human hand preference: Evidence for generation differences, familial resemblance and maternal effects. Behavior Genetics. 1980;10:263–275. doi: 10.1007/BF01067772. [DOI] [PubMed] [Google Scholar]
- McGrew WC, Marchant LF. On the other hand: Current issues in and meta-analysis of the behavioral laterality of hand function in nonhuman primates. Yearbook of Physical Anthropology. 1997;40:201–232. [Google Scholar]
- McKeever WF, Suter PJ, Rich DA. Maternal age and parity correlates of handedness: Gender, but no parental handedness modulation of effects. Cortex. 1995;31:543–553. doi: 10.1016/s0010-9452(13)80065-2. [DOI] [PubMed] [Google Scholar]
- McManus IC, Bryden MP. The genetics of handedness, cerebral dominance and lateralization. In: Rapin I, Segalowitz SJ, editors. Handbook of neuropsychology: Vol 6. Developmental neuropsychology, Part 1. Elsevier; Amsterdam: 1992. pp. 115–144. [Google Scholar]
- Nachshon I, Denno D. Birth order and lateral preferences. Cortex. 1986;22:567–578. doi: 10.1016/s0010-9452(86)80016-8. [DOI] [PubMed] [Google Scholar]
- Nissen HW, Yerkes RM. Reproduction in the chimpanzee: Report on forty-nine births. The Anatomical Record. 1943;86:567–578. [Google Scholar]
- Perelle IB, Ehrman L. An international study of human handedness: The data. Behavior Genetics. 1994;24:217–227. doi: 10.1007/BF01067189. [DOI] [PubMed] [Google Scholar]
- Petridou E, Flytzani V, Youroukas S, Lee IM, Tong D, Trichopoulos D. Birth weight and handedness in boys and girls. Human Biology. 1994;66:1093–1101. [PubMed] [Google Scholar]
- Porac C, Coren S. Lateral preferences and human behavior. Springer; New York: 1981. [Google Scholar]
- Previc FH. A general theory concerning the prenatal origins of cerebral lateralization in humans. Psychological Review. 1991;98:299–334. doi: 10.1037/0033-295x.98.3.299. [DOI] [PubMed] [Google Scholar]
- Provins KA. Handedness and speech: A critical reappraisal of the role of genetic and environmental factors in the cerebral lateralization of function. Psychological Review. 1997;104:554–571. doi: 10.1037/0033-295x.104.3.554. [DOI] [PubMed] [Google Scholar]
- Satz P, Soper HV, Orsini DL. Human hand preference: Three nondextral subtypes. In: Molfese DL, Segalowitz SJ, editors. Brain lateralization in children: Developmental implications. Guilford; New York: 1988. pp. 281–288. [Google Scholar]
- Schwartz M. Left-handedness and high-risk pregnancy. Neuropsychologia. 1977;15:341–344. doi: 10.1016/0028-3932(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Searleman A, Porac C, Coren S. Relationship between birth order, birth stress, and lateral preferences: A critical review. Psychological Bulletin. 1989;105:397–408. doi: 10.1037/0033-2909.105.3.397. [DOI] [PubMed] [Google Scholar]
- Silk J, Short J, Roberts J, Kusnitz J. Gestation length in rhesus macaques (Macaca mulatta) International Journal of Primatology. 1993;14:95–104. [Google Scholar]
- Tan LE, Nettleton NC. Left handedness, birth order, and birth stress. Cortex. 1980;16:363–373. doi: 10.1016/s0010-9452(80)80038-4. [DOI] [PubMed] [Google Scholar]
- Trevarthen C. Lateral asymmetries in infancy: Implications for the development of the hemispheres. Neuroscience and Biobehavioral Reviews. 1996;20:571–586. doi: 10.1016/0149-7634(95)00070-4. [DOI] [PubMed] [Google Scholar]
- Wallis J, Goodall J. Anogenital swelling in pregnant chimpanzees of Gombe National Park. American Journal of Primatology. 1993;31:89–98. doi: 10.1002/ajp.1350310202. [DOI] [PubMed] [Google Scholar]
- Wisniewski AB. Sexually-dimorphic patterns of cortical asymmetry, and the role of sex steroid hormones in determining cortical patterns of lateralization. Psychoneuroendocrinology. 1998;23:519–547. doi: 10.1016/s0306-4530(98)00019-5. [DOI] [PubMed] [Google Scholar]