Abstract
The neurobiology of handedness is still poorly understood in nonhuman primates. Recently, an association between hand preference and precentral gyrus morphology in chimpanzees was reported. The aim of this study was to further evaluate the association between handedness and asymmetries in the precentral gyrus of chimpanzees (Pan troglodytes) and to evaluate the association between hand preference and brain asymmetry using a different approach to the classification of handedness in chimpanzees. The overall results suggest that differences in handedness groups are specific to a region of the precentral gyrus commonly known as the “knob” and that subjects that show different hand preferences differ in brain asymmetries for specific regions of the primary motor cortex. Moreover, using a continuous scale of measurement rather than discrete classification of handedness, significant associations were found between hand use and asymmetries within the precentral gyrus.
Keywords: Handedness, Chimpanzees, Asymmetry, Precentral gyrus, Primary motor cortex
1. Introduction
The neurobiology and evolution of handedness is poorly understood (Hammond, 2002). In humans, handedness often serves as an indirect indicator of hemispheric specialization for language and many have reported the associations between hand use and neuroanatomical asymmetries in cortical areas implicated in linguistic functions including the inferior frontal lobe and planum temporale (Beaton, 1997; Habib, 1989; Moffat, Hampson, & Lee, 1998; Shapleske, Rossell, Woodruff, & David, 1999). However, recent studies using voxel-based morphology measurements have not revealed associations between handedness and asymmetry in either inferior frontal or posterior temporal regions (Good et al., 2001). In addition, increasingly, hand preferences have been associated with neuroanatomical asymmetries in the precental gyrus (Amunts et al., 1996; Foundas, Leonard, & Heilman, 1995; Hammond, 2002). The evidence of significant associations in brain regions outside of the classically defined language regions raises some questions regarding the causal relationship between handedness and hemispheric specialization for language.
Historically, population-level behavioral and neuroanatomical asymmetries have been considered hallmarks of human evolution (Warren, 1980). However, recent studies have documented evidence of population-level behavioral asymmetries in a host of nonhuman vertebrate species (Rogers & Andrew, 2002). With specific reference to primates, there is increasing evidence of population-level handedness in prosimians (Ward, Milliken, & Stafford, 1993), Old and New World monkeys (Hook-Costigan & Rogers, 1997; Spinozzi, Castornina, & Truppa 1998; Vauclair Mequerditchian, & Hopkins, 2005; Westergaard, Kuhn, & Suomi, 1998) and great apes (Hopkins, Stoinski, Lukas, Ross, & Wesley, 2003; Hopkins, Wesley, Izard, Hook, & Schapiro, 2004). Moreover, evidence of population-level neuroanatomical asymmetries have been reported in great apes. For example, great apes show a leftward asymmetry in the planum temporale (Cantalupo, Pilcher, & Hopkins, 2003; Gannon, Holloway, Broadfield, & Braun, 1998; Hopkins, Marino, Rilling, & MacGregor, 1998), inferior frontal gyrus (Cantalupo & Hopkins, 2001, Cantalupo, Rodes, Hegarty, Freeman, & Hopkins, 2006)and sylvian fissure length (Hopkins, Pilcher, & MacGregor, 2000). In addition, great apes show a right-frontal, left-occipital torque asymmetry in cortical volume (Pilcher, Hammock, & Hopkins, 2001). Collectively, these data strongly challenge the long held belief that population-level asymmetries are specific to Hominid evolution.
Whether hand preferences are associated with neuroanatomical asymmetries has been less studied in nonhuman primates but some evidence has been reported. In squirrel monkeys, greater neural activity in the motor cortex is found in the hemisphere contralateral to the preferred hand (Nudo, Jenkins, Merzenich, Prejean, & Grenda, 1992). In capuchin monkeys, asymmetries in the dorsal portion of the precental gyrus are correlated with hand preferences for coordinated bimanual actions (Phillips & Sherwood, 2005). Similarly, in chimpanzees, hand preferences are associated with asymmetries in the “knob”, a region of the precentral gyrus but not with asymmetries in either the inferior frontal gyrus or planum temporale (Hopkins & Cantalupo, 2004).
One purpose of this study was to further evaluate the association between handedness and asymmetries in the precentral gyrus. One limitation of the Hopkins and Cantalupo (2004) study was that measurements of neuroanatomical asymmetries within the precentral gyrus were restricted to a specific landmark, referred to as the “knob”. This region presumably represents where the hand is represented on the motor strip but hand preferences were never compared from different regions within the precentral gyrus. In other words, if the “knob” represents where the hand is located in the motor strip and this corresponds to the specific neurobiological correlate for handedness in chimpanzees, then the association between hand preferences and precentral gyrus asymmetries should be specific to this region of the motor cortex. To test this hypothesis, the depth of the central sulcus (CS) was measured from the most dorsal to most ventral section and divided into four evenly spaced sections. Asymmetries from each region were computed and compared between right-, left- and ambidextrous chimpanzees.
A second purpose of this study was to evaluate the association between hand preference and brain asymmetry using a different approach to the classification of handedness in chimpanzees. In the previous work by Hopkins and Cantalupo (2004), right- and left-handed subjects were compared for three different measures including coordinated bimanual actions, bimanual feeding and simple reaching. For each measure, left-handed, right-handed and non-preferent animals were compared for each brain region. This approach is quite different than that typically used with human subjects in which a single, unitary measure of hand preference is derived based on multiple measures of hand use. In this study, rather than compare subjects on specific measures of hand preference, we assessed handedness for two measures and then classified the chimpanzee as consistently right-handed, consistently left-handed or ambidextrous based on their hand use across the two measures. This approach is more similar to that used with human subjects because there is a true “ambidextrous” group (inconsistent hand use across measures) rather than ambiguously-handed subjects (inconsistent hand use for the same hand preference measure) (see Satz, Soper & Orsini, 1988). We hypothesized that ambidextrous subjects would show no neuroanatomical asymmetries whereas right- and left-handed chimpanzees would show opposite brain asymmetry patterns.
2. Method
2.1. Subjects
Magnetic resonance images (MRI) were collected in a sample of 60 chimpanzees (Pan troglodytes) including 33 females and 27 males ranging in age from 8 to 49 years (Mean = 22.07, s.d. = 11.63). All the chimpanzees are members of a captive colony housed at Yerkes National Primate Research Center (YNPRC) in Atlanta, Georgia. Nineteen of the brains were scanned post-mortem, while the other 51subjects were alive and healthy at the time of the scan. Ages ranged from 11 to 48 years (Mean = 26.60 years) for the post-mortem brains and 8–47 years (Mean = 20.20 years) for the in vivo scans.
2.2. Image collection and procedure
Prior to scanning, the subjects were immobilized with ketamine injection (2–6 mg/kg) and subsequently anesthetized with propofol (10 mg/kg/h) following standard veterinary procedures used at the YNPRC. The subjects remained sedated for the duration of the scans as well as the time needed for transport between YRPRC and scanner location (total time approximately 1 h). After completing the MRI scan, the nonhuman primate subjects were returned to Yerkes and were temporarily housed in a single cage for 6–12 h to allow the effects of the anesthesia to wear off before being returned to their home cage and cagemates.
At the MRI facility, the animals were placed in the scanner chamber and their heads were fitted inside the head coil. The cadaver brains were placed inside the human-knee coil with the dorsal side up. Scan duration ranged between 40 and 80 min as a function of brain size. This project involved using two MRI machines (Phillips, Model NT), each with 1.5-T superconducting magnets. For all subjects, T1-weighted images were collected in the axial plane using a gradient echo protocol (pulse repetition = 19.0 ms, echo time = 8.5 ms, slice thickness 1.2 mm, slice overlap = .6 mm, number of signals averaged = 8 and a 256 × 256 matrix). These scan parameters were based on preliminary studies and provided excellent resolution of the brain areas of interest. The raw images were reformatted into the different planes using multiplanar formatting software (ANALYZE).
Central sulcus (CS)
The depth of the CS was measured in serial 1 mm slices in the axial plane following procedures previously used in human and ape brain specimens (Hopkins & Pilcher, 2001; Yousry et al., 1997). For each slice and hemisphere, a line was traced along the fissure from the most lateral to most medial points. The depths of the CS were determined from the most dorsal to most ventral slice in the axial plane. The total number of slices was divided by four to divide the CS into four evenly spaced sections from the most dorsal to ventral regions (referred to as CS1, CS2, CS3, & CS4). Individual asymmetry quotients were derived for each section of the CS following the formula [AQ = (R−L)/((R+L) * 0.5)]. Positive values indicated right hemisphere biases and negative values reflected left hemisphere biases. MD traced all of the brains and was blind to the handedness of the subjects. The CS was re-traced in 10 subjects by MD to assess intra-rater reliability in the measurement of each hemisphere. Test-retest correlations in total CS volume for the right (r = .950, df = 8, p < .001) and left (r = .806, df = 8, p < .005) hemisphere were positive and significant indicating good reliability.
2.3. KNOB Asymmetry
Within the CS, we also evaluated whether any potential significant differences between handedness groups were directly related to the brain region corresponding to the KNOB. The KNOB is a horizontal epsilon or inverted omega that projects into the post central gyrus was traced on each image (see Fig. 1). The dorsal and ventral edges of the knob served as the markers for defining the boundaries of the area. The total depths of the CS from all slices in which the knob was present were summed and used to derive a volume of this CS region for each hemisphere.
Fig. 1.
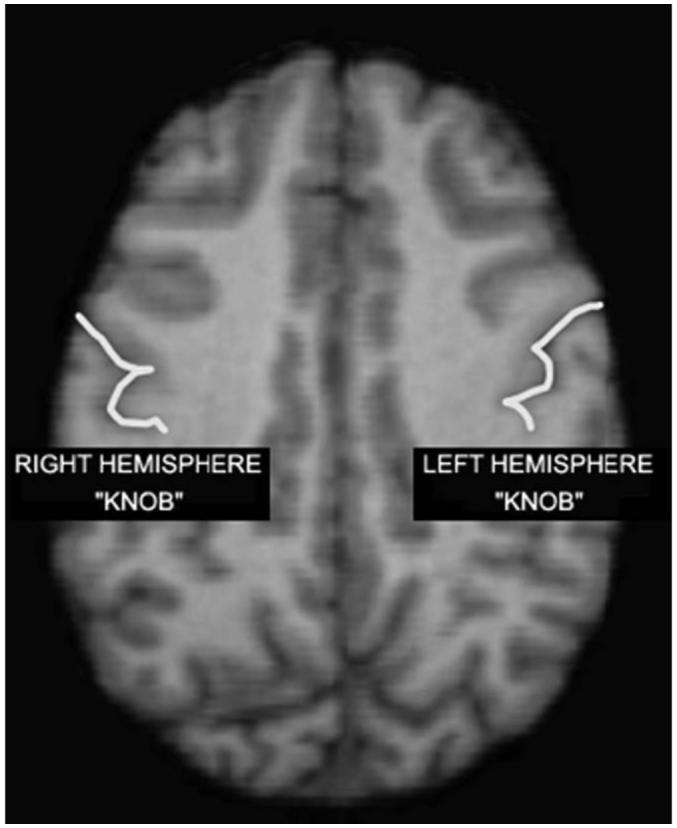
Axial view of an MRI scan of a chimpanzee brain. The white lines indicate the central sulcus (CS) and the epsilon or omega shaped region with the CS represents the KNOB.
2.4. Behavioral measures of hand use
Hand preferences were derived from two measures including bimanual feeding and a task assessing hand use for coordinated bimanual actions referred to as the TUBE task. In our previous studies (Hopkins & Cantalupo, 2004), we assessed the association between brain asymmetry and three measures of hand use including coordinated bimanual actions, bimanual feeding and simple reaching. The simple reaching measure was dropped in this study because it positively correlated with the bimanual feeding task and it failed to correlate with KNOB asymmetries in our previous studies. In addition, simple reaching is influenced by situational factors and we sought to base handedness classifications on measures that required subject to make a choice of which hand to use when both hands had to engage in specific functions. A brief description of each task is provided below.
2.5. Bimanual feeding (FEED)
Each afternoon, the primates housed at the YNPRC receive fruits and vegetables as part of their daily diet. Each subject usually receives two oranges, one banana, some celery stalks, and/or carrots. Once retrieving the food, the subjects typically move to a seating place and consume the food. The chimpanzees typically hold the extra pieces of food with one hand and feed with the opposite hand. Hand use was recorded when the subjects were feeding with one hand for a minimum duration of 3 s and the non-feeding hand was holding the remaining portions of food (Hopkins, 1994). The dominant hand was recorded as the one feeding. A minimum of 50 responses were obtained from each subject.
2.6. Coordinated bimanual actions (TUBE)
The second handedness measure was a task requiring bimanual coordinated actions, referred to as the TUBE task (Hopkins, 1995). For the TUBE task, peanut butter is smeared on the inside edges of poly-vinyl-chloride (PVC) tubes approximately 15 cm in length and 2.5 cm in diameter. Peanut butter is smeared on both ends of the PVC pipe and is placed far enough down the tube such that the subjects cannot lick the contents completely off with their mouths but rather must use their fingers to remove the substrate. The PVC tubes were handed to the subjects in their home cages and a focal sampling technique was used to collect individual data from each subject. The hand of the finger used to extract the peanut butter was recorded as either right or left by the experimenter. Each time the subjects reached into the tube with their finger, extracted peanut butter and brought it to their mouth, the hand used was recorded as left or right.
2.7. Data analysis
For the two handedness tasks, binomial z-scores were calculated for each subject based on the frequency of left and right hand use for all assessments of hand use. Subjects with z-scores greater than 1.95 or less than −1.95 were classified as right- and left-handed. Subjects with z-scores between −1.96 and 1.96 were classified as non-preferent. Based on the z-scores for each measure, subjects were classified as right-handed if they preferred their right hand for both measures or preferred the right for one and showed no bias for the other (n = 26). Subjects were classified as left-handed if they preferred to use their left hand for both measures or preferred their left hand for one and had no bias for the second measure (n = 18). Chimpanzees that showed a right hand bias on one task and a left hand bias on the other or chimpanzees that showed no bias on either task (this was rare, n = 3) were classified as ambidextrous (n = 16). All analyses adopted an alpha of p < .05 as the level of significance. Post hoc tests, when necessary, were conducted using Tukey's honestly significant difference (HSD) with p < .05.
3. Results
The four AQ scores from each region of the CS served as dependent variables in a multiple analysis of variance (MANOVA). Sex (male, female) and handedness (left-handed, ambidextrous, right-handed) served as independent variables. A significant main effect for handedness was found for the MANOVA F(4, 52) = 3.15, p < .03. Subsequent univariate F-tests revealed a significant effect of handedness for CS2 only F(2, 54) = 5.68, p < .006. The mean AQ scores for each handedness group and each central sulcus region are shown in Fig. 2. Post hoc analysis indicated that the mean AQ scores for right-handed chimpanzees were significantly lower than left-handed but not ambidextrous chimpanzees. No significant difference was found between left- and ambidextrous chimpanzees.
Fig. 2.
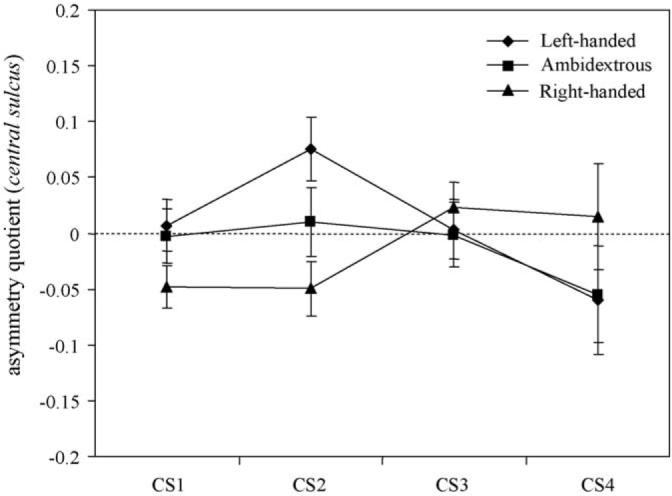
Mean asymmetry quotients (AQ's) and the standard errors for each region of the CS in relation to the handedness of the chimpanzees. C1–C4 represent dorsal to ventral measures.
In the next analysis, we determined whether the observed handedness effects for CS2 were primarily due to asymmetries in the KNOB. Separate volumes of the KNOB for the left and right hemisphere were calculated and a subsequent AQ value was derived for the slices corresponding to the KNOB following the previously described formula. The KNOB-AQ was then compared between handedness groups and sexes using an analysis of variance. A significant main effect for handedness was found F(2, 54) = 3.39, p < .05. Post hoc analysis indicated that the mean KNOB-AQ scores for right-handed chimpanzees (Mean = −.227) was significantly lower than left-handed (Mean = .175) but not ambidextrous individuals (Mean = .047).
3.1. Intercorrelations among measures
As an alternative means of assessing the association between hand use and brain asymmetry, we correlated handedness as derived on a continuous scale of measurement with the AQ scores for the entire CS, each region of the CS and the KNOB-AQ values using a Pearson product moment correlation. Handedness was derived for this analysis by calculating a handedness index for each measure following the formula [HI = (#R − #L)/#R+#L)] with #R and #L referring to the frequency of left and right hand responses. We then averaged the HI scores for FEED and TUBE tasks to derive an average handedness index (MEAN-HI). Significant negative associations were found between the MEAN-HI and CS1 and CS2. Similarly, significant negative association were found between the HI scores for the TUBE task and CS1 and CS2. No significant associations were found between the HI scores for the FEED task and any of the CS regions.
4. Discussion
The results of this study revealed three main findings. First, right-, left- and ambidextrous subjects significantly differ in brain asymmetries for specific regions of the primary motor cortex. Second, differences in handedness group are specific to a region of the precentral gyrus that corresponds to the “knob” as has been previously described in human and nonhuman primates (Hopkins & Cantalupo, 2004; Yousry et al., 1997). Third, using a continuous scale of measurement rather than discrete classification of handedness, significant associations were found between hand use and asymmetries within the precentral gyrus.
In general, the analysis of variance and correlation analyses revealed similar findings, although there were some differences depending on how handedness was characterized. The most consistent result revealed from the various analyses was the association between handedness and CS2 asymmetry; however, the significant correlation found between CS1 and the continuous measure of handedness is a curious finding. Because CS1 is the most dorsal region of the precentral gyrus, motor representation of the lower limbs and trunk are present in these regions yet they correlated with the handedness of the apes. This may suggest that the entire dorsal portion of the CS organizes itself around handedness or, alternatively, there are inherent associations among hand preference and other lateral biases involving the trunk or the lower limbs. In humans, footedness is mildly associated with handedness (Gabbard & Iteya, 1996; Porac & Coren, 1981; Reiss & Reiss, 1997). Other studies suggest a possible link between handedness and whole-body turning preference in humans (Bracha, Seitz, Otemma, & Glick, 1987; Mohr, Landis, Bracha, & Brugger, 2003) and in prosimians (Ward & Cantalupo, 1997). These findings might account for the association between handedness and both regions CS1 and CS2 in chimpanzees. In the absence of footedness data in chimpanzees, this observation is speculative but certainly testable in the apes, as well as humans (Table 1).
Table 1.
Partial correlation coefficients between each handedness measure and central sulcus asymmetry
| Region of the central sulcus |
||||
|---|---|---|---|---|
| CS1 | CS2 | CS3 | CS4 | |
| Variable | ||||
| MEAN-HI | −.327 | −.366 | .133 | .113 |
| TUBE | −.334 | −.320 | .062 | .058 |
| EFED | −.114 | −.229 | .116 | .123 |
Bold indicates p < .05.
In conclusion, the results of this study provide further evidence of an association between handedness and asymmetries in the primary motor cortex of chimpanzees. Chimpanzee hand preferences correlate with the depth of the CS, particularly in the more dorsal regions of the motor cortex, a finding consistent with recent studies in humans (Amunts et al., 1996; Foundas et al., 1995) and capuchin monkeys (Phillips & Sherwood, 2005). Moreover, the strongest and most consistent association between handedness and brain asymmetry were linked to the KNOB, a region of the precentral gyrus corresponding to the hand region (at least in humans). The association between hand preference and the KNOB suggests that differential use of one hand over the other has a significant influence on the organization of the hand-specific region of the motor cortex in chimpanzees. Further research should focus on identifying whether preferences manifest in other limbs might map to asymmetries in specific regions of the motor cortex.
Acknowledgements
This work was supported in part by NIH grants RR-00165, NS-42867, NS-36605 and HD-38051. The Yerkes Center is fully accredited by the American Association for Accreditation of Laboratory Animal Care and APA guidelines for the ethical treatment of animals were adhered to during all aspects of this study. Special thanks to the veterinary staff for assisting in the care of the animals during scanning.
References
- Amunts K, Schlaug G, Schleicher A, Steinmutz H, Drabinghaus A, Roland P, Zilles K. Asymmetry in the human motor cortex and handedness. NeuroImage. 1996;4:216–222. doi: 10.1006/nimg.1996.0073. [DOI] [PubMed] [Google Scholar]
- Beaton AA. The relation of planum temporale asymmetry and morphology of the corpus callosum to handedness, gender and dyslexia: a review of the evidence. Brain and Language. 1997;60:255–322. doi: 10.1006/brln.1997.1825. [DOI] [PubMed] [Google Scholar]
- Bracha HS, Seitz DJ, Otemaa J, Glick SD. Rotational movement (circling) in normal humans: sex difference and relationship to hand, foot and eye preference. Brain Research. 1987;411:231–235. doi: 10.1016/0006-8993(87)91074-2. [DOI] [PubMed] [Google Scholar]
- Cantalupo C, Hopkins WD. Asymmetric Broca's area in great apes. Nature. 2001;414:505. doi: 10.1038/35107134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantalupo C, Pilcher D, Hopkins WD. Are asymmetries in Sylvian fissure length associated with the planum temporale? Neuropsychologia. 2003;41:1975–2181. doi: 10.1016/s0028-3932(02)00288-9. [DOI] [PubMed] [Google Scholar]
- Cantalupo C, Rodes WM, Hegarty JP, Freeman H, Hopkins WD. Macrostructural asymmetry of Broca's area region in the great ape brain: is morphological variability an insurmountable problem? submitted for publication. [Google Scholar]
- Foundas A, Leonard C, Heilman K. Morphological cerebral asymmetries and handedness: the pars triangularis and planum temporale. Archives of Neurology. 1995;52:501–508. doi: 10.1001/archneur.1995.00540290091023. [DOI] [PubMed] [Google Scholar]
- Gabbard C, Iteya M. Foot laterality in children, adolescents and adults. Laterality. 1996;1:199–205. doi: 10.1080/713754236. [DOI] [PubMed] [Google Scholar]
- Gannon PJ, Holloway RL, Broadfield DC, Braun AR. Asymmetry of chimpanzee planum temporale: humanlike pattern of Wernicke's brain language area homologue. Science. 1998;279:220–222. doi: 10.1126/science.279.5348.220. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnstrude I, Ashburner J, Henson RNA, Friston KJ, Frackowiak RSJ. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal human brains. NeuroImage. 2001;14:685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- Habib M. Anatomical asymmetries of the human cerebral-cortex. International Journal of Neuroscience. 1989;47:67–79. doi: 10.3109/00207458908987419. [DOI] [PubMed] [Google Scholar]
- Hammond G. Correlates of human handedness in primary motor cortex: a review and hypothesis. Neuroscience and Biobehavioral Reviews. 2002;26:285–292. doi: 10.1016/s0149-7634(02)00003-9. [DOI] [PubMed] [Google Scholar]
- Hook-Costigan MA, Rogers LJ. Hand preferences in New World primates. International Journal of Comparative Psychology. 1997;9:173–207. [Google Scholar]
- Hopkins WD, Cantalupo C. Handedness in chimpanzees (Pantroglodytes) is associated with asymmetries of the primary motor cortex but not with homologous language areas. Behavioral Neuroscience. 2004;118:1176–1183. doi: 10.1037/0735-7044.118.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD. Hand preferences for bimanual feeding in 140 captive chimpanzees (Pan troglodytes): rearing and ontogenetic factors. Developmental Psychobiology. 1994;27:395–407. doi: 10.1002/dev.420270607. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Hand preferences for a coordinated bimanual task in 110 chimpanzees: Cross-sectional analysis. Journal of Comparative Psychology. 1995;109:291–297. doi: 10.1037/0735-7036.109.3.291. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Pilcher DL, MacGregor L. Sylvian fissure asymmetries in nonhuman primates revisited: a comparative MRI study. Brain Behavior and Evolution. 2000;56:293–299. doi: 10.1159/000047213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Pilcher DL. Neuroanatomical localization of the motor hand area using magnetic resonance imaging: The left hemisphere is larger in great apes. Behavioral Neuroscience. 2001;115:1159–1164. [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Marino L, Rilling J, MacGregor L. Planum temporale asymmetries in great apes as revealed by magnetic resonance imaging (MRI) NeuroReport. 1998;9:2913–2918. doi: 10.1097/00001756-199808240-00043. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Stoinski T, Lukas K, Ross S, Wesley MJ. Comparative assessment of handedness for a coordinated bimanual task in chimpanzees (Pan), gorillas (Gorilla), and orangutans (Pongo) Journal of Comparative Psychology. 2003;117:302–308. doi: 10.1037/0735-7036.117.3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Wesley MJ, Izard MK, Hook M, Schapiro SJ. Chimpanzees are predominantly right-handed: replication in three colonies of apes. Behavioral Neuroscience. 2004;118:659–663. doi: 10.1037/0735-7044.118.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat SD, Hampson E, Lee DH. Morphology of the planum temporale and corpus callosum in left handers with evidence of left and right hemisphere speech representation. Brain. 1998;121:2369–2379. doi: 10.1093/brain/121.12.2369. [DOI] [PubMed] [Google Scholar]
- Mohr C, Landis T, Bracha HS, Brugger P. Opposite turning behavior in right-handers and non-right-handers suggests a link between handedness and cerebral dopamine asymmetries. Behavioral Neuroscience. 2003;117:1448–1452. doi: 10.1037/0735-7044.117.6.1448. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Jenkins WM, Merzenich MM, Prejean T, Grenda R. Neurophysiological correlates of hand preference in primary motor cortex of adult squirrel monkeys. The Journal of Neuroscience. 1992;12:2918–2947. doi: 10.1523/JNEUROSCI.12-08-02918.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips K, Sherwood CS. Primary motor cortex asymmetry correlates with handedness in capuchin monkeys (Cebus apella) Behavioral Neuroscience. 2005;119:1701–1704. doi: 10.1037/0735-7044.119.6.1701. [DOI] [PubMed] [Google Scholar]
- Pilcher DL, Hammock ED, Hopkins WD. Cerebral volu-metric asymmetries in nonhuman primates: a magnetic resonance imaging study. Laterality. 2001;6:165–179. doi: 10.1080/13576500042000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porac C, Coren S. Lateral preferences and human behavior. Springer; New York: 1981. [Google Scholar]
- Reiss M, Reiss G. Lateral preferences in a German population. Perceptual and Motor Skills. 1997;85:569–574. doi: 10.2466/pms.1997.85.2.569. [DOI] [PubMed] [Google Scholar]
- Rogers LJ, Andrew R. Comparative vertebrate lateralization. Oxford University Press; Oxford: 2002. [Google Scholar]
- Satz P, Soper HV, Orsini DL. Human hand preference: three nondextral subtypes. In: Molfese DL, Segalowitz SJ, editors. Brain lateralization in children: developmental implications. The Guilford Press; New York: 1988. pp. 281–288. [Google Scholar]
- Shapleske J, Rossell SL, Woodruff PWR, David AS. The planum temporale: a systematic, quantitative review of its structural, functional and clinical significance. Brain Research Reviews. 1999;2:26–49. doi: 10.1016/s0165-0173(98)00047-2. [DOI] [PubMed] [Google Scholar]
- Spinozzi G, Castornina MG, Truppa V. Hand preferences in unimanual and coordinated-bimanual tasks by tufted capuchin monkeys (Cebus apella) Journal of Comparative Psychology. 1998;112:183–191. [Google Scholar]
- Vauclair J, Mequerditchian A, Hopkins WD. Hand preferences for unimanual and coordinated bimanual tasks in baboons (Papio anubis) Cognitive Brain Research. 2005;25:210–216. doi: 10.1016/j.cogbrainres.2005.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JP, Cantalupo C. Origins and functions of laterality: Interactions of motoric systems. Laterality. 1997;2:279–303. doi: 10.1080/713754271. [DOI] [PubMed] [Google Scholar]
- Ward J, Milliken G, Stafford D. Patterns of lateralized behavior in prosimians. In: Ward J, Hopkins W, editors. Primate laterality: current behavioral evidence of primate asymmetries. Springer-Verlag; New York: 1993. pp. 43–71. [Google Scholar]
- Warren JM. Handedness and laterality in humans and other animals. Physiological Psychology. 1980;8:351–359. [Google Scholar]
- Westergaard GC, Kuhn HE, Suomi SJ. Bipedal posture and hand preference in humans and other primates. Journal of Comparative Psychology. 1998;112:56–63. doi: 10.1037/0735-7036.112.1.55. [DOI] [PubMed] [Google Scholar]
- Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997;120:141–157. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]


