Abstract
Objective:
To assess knowledge of phosphorus compared with other nutrients in patient undergoing maintenance dialysis (MD).
Design:
We compared knowledge of phosphorus versus other nutrients important to the MD diet (potassium, sodium, protein) in hemodialysis (HD) and peritoneal dialysis (PD) patients. We further measured gender, age, education level and functional health literacy to assess correlations in patient nutrient knowledge. Nutrient knowledge was measured using a 25-item chronic kidney disease knowledge assessment tool for nutrition (CKDKAT-N), and functional health literacy was measured using the short-form of the test of functional health literacy in adults (STOFHLA).
Setting:
Patients receiving maintenance outpatient HD or PD at the University of Wisconsin Hospital and Clinics Kidney Clinic, Wisconsin Dialysis Incorporated.
Main outcome measure:
Phosphorus knowledge versus knowledge of potassium, sodium and protein.
Results:
Forty-seven MD patients participated in the study (29 HD, 18 PD, 30 males and 17 females, average age 58.6 (SD 13.8) years, average grade level 1.4 (SD 2.6) years of post-secondary education). 35 participants had adequate, 4 marginal and 8 inadequate health literacy. CKDKAT-N scores ranged from 6 to 21 of 25 items, mean score of 13 (SD 2.91). Knowledge of phosphorus compared with knowledge of other nutrients was poor (0.38 versus 0.72, p = 0.003). Comparing HD to PD patient knowledge, both phosphorus (0.37 versus 0.42, p=0.231) and other nutrients (0.69 versus 0.80, p=0.115) were the same.
Conclusion:
Despite regular dietary instruction, patients undergoing MD have a poor knowledge of dietary phosphorus content, compared with knowledge of other nutrients important in chronic kidney disease. Interestingly, there was no difference in nutrition knowledge when comparing PD and HD patients, despite differences in education level and health literacy between the groups.
Keywords: Dietary Education, Dialysis, Phosphorus, Health Literacy, Potassium, Sodium, Protein
Introduction
The progression of chronic kidney disease (CKD) to end-stage renal disease is associated with poor regulation of serum phosphorus and calcium, despite the institution of renal replacement therapy. Phosphorus is increasingly recognized as playing an important role in not only the bone disease of CKD, but cardiovascular morbidity as well.[1] The relationship between elevated phosphorus levels and morbidity and mortality in patients with ESRD is powerful,[2] and hyperphosphatemia is independently associated with a variety of poor cardiovascular outcomes, including death.[3-5]
Along with dietary restriction of phosphorus intake, the use of phosphate binder medications, such as calcium carbonate, calcium acetate, sevelamer hydrochloride, and lanthanum carbonate, is the mainstay for the treatment of hyperphosphatemia in maintenance dialysis (MD) patients. Despite these measures, phosphorus is difficult to control, and Goldfarb recently suggests that dietary manipulation is an underappreciated tool for reducing hyperphosphatemia.[6] Although many dialysis centers employ dietary counseling and instruction by a registered dietician as a means to help control dietary phosphorus intake, MD patients often have difficulty understanding, assimilating, and implementing dietary recommendations. In addition, there is conflicting data regarding the utility of educational counseling to alter patient knowledge of dietary phosphorus.[7-9]
Low health literacy (the ability to read, understand, and act on health care information) affects more than 90 million adults in the US[10-12] and is associated with a variety of adverse outcomes, including sub-optimal chronic disease self-management.[13-17] Although no definitive association between these variables has been established, Jain et al recently reported that of 92 MD patients, no association between health literacy and serum phosphate was identified.[18] Moreover, there was a trend toward higher serum phosphate levels in patients with greater levels of functional health literacy, suggesting that the challenges of controlling dietary phosphorus intake may transcend parameters such as literacy and education levels alone.
The evaluation of patient knowledge of phosphorus in the hemodialysis (HD) and peritoneal (PD) dialysis population is poorly studied. MD patients are typically given dietary instruction regarding food choices, phosphorus intake, as well as sodium, potassium and fluid restriction. Despite this, knowledge of phosphorus remains poor, especially in HD patients.[7, 8, 19] The purpose of our study was to assess the knowledge of phosphorus compared with other nutrients (sodium, potassium, protein) in the MD population. We further sought to assess for differences in nutrition knowledge in patients utilizing different dialysis modalities (PD vs. HD). We created a Chronic Kidney Disease Knowledge Assessment Tool for Nutrition, (CKDKAT-N) in order to evaluate these differences. Finally, we sought to exclude the possibility that a lack of formal education or low health literacy levels could explain differences in either peritoneal dialysis or HD patient nutrition knowledge. We hypothesized that knowledge of phosphorus by MD patients is poor compared to other nutrients, that higher levels of education and health literacy would not improve this knowledge, and that PD patients would have better knowledge compared to HD patients.
Methods
Patients receiving PD or HD at the University of Wisconsin Hospitals and Clinics Kidney Clinic, Wisconsin Dialysis Inc. were approached to participate in the study. At this center, both PD and HD patients are given once-monthly dietary instruction customized by the dietician according to the patient's laboratory data and fluid-volume status. Following informed consent, demographic information, including age, gender, dialysis modality, length of dialysis therapy, and educational level was collected. The patients were tested for nutrition knowledge and health literacy either during their PD clinic appointment, or during or after their HD session. The CKDKAT-N was used to evaluate MD patient nutrition knowledge. This questionnaire is composed of 25 multiple-choice questions reflecting knowledge of 4 nutrients relevant to MD patients: phosphorus, protein, sodium, and potassium. Fifteen questions concern phosphorus, and the remaining 10 test the domains of protein, sodium, and potassium. Test questions are based on guidelines from the National Kidney Foundation[20] and the Kidney Disease Quality Outcomes Initiative (KDOQI).[21]Nutrient content of foods was determined from the United States Department of Agriculture (USDA) Nutrient Data Laboratory's National Nutrient Database ,[22] with portion size specified in the questions. The study was approved by the University of Wisconsin Health Sciences Institutional Review Board.
Health literacy levels were assessed using the short form of the test of functional health literacy in adults (S-TOFHLA).[23] An abbreviated version of the TOFHLA, the S-TOFHLA categorizes individuals as having adequate, marginal or inadequate health literacy, is available in English and Spanish, and has been used in patients with a variety of chronic diseases.[24] A 36-item multiple-choice test, it utilizes short reading passages with multiple-choice answers, and is administered in 7 minutes. Results are scored on a scale of 0-36, with 0-16 identifying inadequate health literacy, 17-22 marginal health literacy, and 23 to 36 adequate health literacy.
Test scores and nutrition knowledge were recorded into a database. The questions in each subset of phosphorus, sodium, potassium, and protein knowledge were recorded, and composite scores of knowledge of phosphorus and knowledge of other nutrients were determined.
Analysis of mean values between the groups for age (years), education (highest grade levels), dialysis vintage (months), total score for the CKD-KAT and subset scores for knowledge of phosphorus nutrition, and knowledge of other nutrients (sodium, potassium, and protein) were compared using unpaired t-tests. Gender distribution and health literacy level, by ranking of adequate, marginal, or inadequate, were compared descriptively.
Results
Forty-seven MD patients participated in the study (29 HD, 18 PD- Table). Overall, there were 30 males and 17 females, with an average age of 58.6 years (range 24 to 84, SD 13.8). Average grade level completed was 1.4 years of post-secondary education (mean grade 13.4, range 6 to 20, SD 2.6). By S-TOFHLA ranking 35 participants had adequate, 4 marginal and 8 inadequate health literacy. CKDKAT-N scores ranged from 6 to 21 of 25 items, with a mean score of 13 (SD 2.91).
Table 1.
Patient Characteristics
| Hemodialysis | Peritoneal Dialysis | |
|---|---|---|
| Number | 29 | 18 |
| Average age | 60.8 | 54.9 |
| Male | 19 | 11 |
| Female | 10 | 7 |
| Grade level completed (average) |
12.8 | 14.4 |
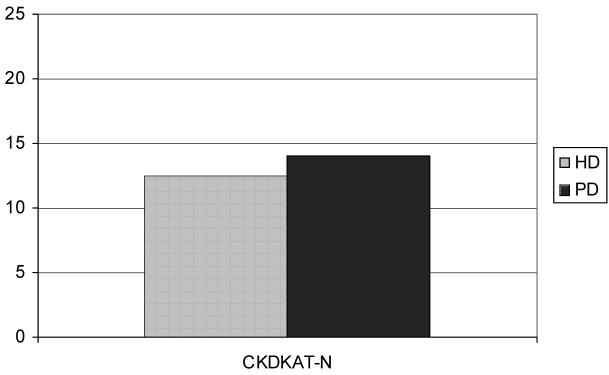
The HD group consisted of 19 males and 10 females, with an average age of 60.8 (range 34 to 84 years, SD 14.2) and average grade level completed of 0.8 years of post-secondary education (mean grade 12.8, range 6 to 20, SD 2.8). S-TOFHLA ranking of the HD patients revealed that 18 had adequate, 4 marginal, and 7 inadequate health literacy (figure 1). CKDKAT-N scores for the HD group ranged from 6 to 19 out of 25 questions, with a mean score of 12.5 (SD 2.86) (figure 2).
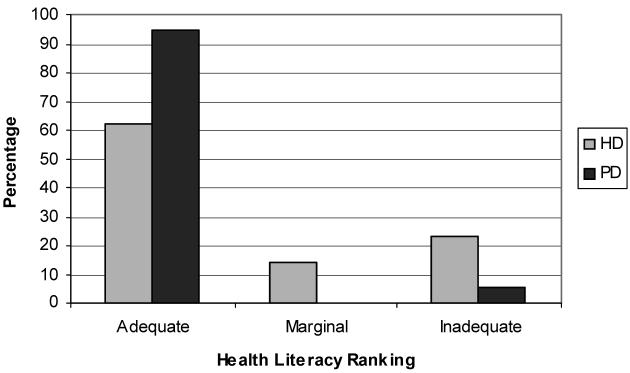
Figure 1.
S-TOFHLA Ranking Distribution. Of the HD, 18 had adequate, 4 marginal, and 7 inadequate health literacy. Of the PD patients, 17 had adequate and 1 had inadequate health literacy.
Figure 2.
CKDKAT-N scores are the same for the PD patients and HD patients (mean 14 versus 12.5, p = 0.082)
The PD group consisted of 11 males and 7 females, with an average age of 54.9 (range 24 to 72 years, SD 12.6, p = 0.154 compared with HD), and average grade level completed of 2.4 years of post-secondary education (mean grade 14.4, range 12 to 18, SD 1.89, p = 0.029 compared with HD). S-TOFHLA ranking of the PD patients revealed that 17 had adequate and 1 had inadequate health literacy (figure 1). The CKDKAT-N scores for the PD group ranged from 7 to 21, with a mean score of 14 (SD 2.8, p = 0.082 compared with HD) (figure 2).
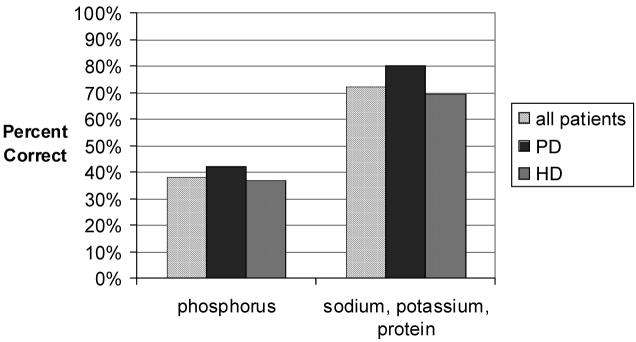
Knowledge of phosphorus was compared with knowledge of the other nutrients (0.38 versus 0.72, p = 0.003), and both phosphorus and other nutrient knowledge was compared between HD and PD patients, as was knowledge of the other nutrients (phosphorus knowledge- 0.37 versus 0.42, p=0.231; other nutrient knowledge- 0.69 versus 0.8, p=0.115) (figure 3).
Figure 3.
Phosphorus knowledge in all patients (hatched white bar) is worse than knowledge of other nutrients (p=0.003). The knowledge is equally poor in PD patients (dark gray bar) as compared to HD patients (light gray bar), p = 0.231. The PD and HD patients had far better knowledge of other nutrients, and scores were NS between groups in these categories, p = 0.115.
Discussion
Despite widespread awareness of the increased morbidity and mortality associated with elevated levels of serum phosphorus in patients undergoing MD, hyperphosphatemia remains common in this population. Slinin et al, in a recent cohort analysis of almost 15,000 patients from the Unites States Renal Data System (USRDS), found phosphorus levels greater than current guideline recommendations in over half of individuals. Furthermore, the adjusted hazard ratio for a cardiovascular event in this group rose as serum phosphorus increased above 5.3 mg/dl, and all cause mortality rose as serum phosphorus levels increased above 6.4 mg/dl.[25] In another large cohort study, Block demonstrated a similar increased relative risk of death for serum phosphorus above 5.0.[1] This poor control of serum phosphorus persists despite the implementation of phosphate binders as standard therapy in dialysis patients. In the DOPPS cohort from 1996 to 2001, although 81 percent of the patients were treated with a binder medications, 52 percent had phosphorus levels above the target range.[9] Results of the CARE study do suggest that although poor phosphorus control has improved with the introduction of calcium acetate and sevelamer hydrochloride as binders, there are still large numbers of patients with poorly controlled phosphorus.[26]
Given this, dietary phosphorus restriction remains an essential component of the effort to control serum phosphorus. Dietary counseling is routinely used to educate patients regarding the phosphorus content of foods and to reinforce adherence and educate patients regarding the consequences of high serum phosphorus. Studies that have evaluated these methods have demonstrated conflicting data regarding the utility of educational counseling. Cupisti et al showed that HD patients with high serum phosphorus (> 5.5 mg/dL) were able to decrease dietary phosphorus and calcium intake, and had improved calcium-phosphate product after intensive dietary intervention and counseling.[7] Similarly, Ford et al showed that serum phosphorus levels and the calcium-phosphorus product were improved after six months when patients were given a 20-30 minute diet education program each month specifically targeting phosphorus.[8] Conversely, Schlatter et al found that a teaching intervention in a group of 29 hemodialysis patients with high phosphorus levels had a weak affect on serum phosphorus levels, despite an increase in phosphorus knowledge. In their analysis, the relationship between a decrease in serum phosphorus and increases in phosphorus knowledge did not reach statistical significance after a single education session utilizing a teaching booklet, osteodystrophy tool, and medication diary.[9] Some authors have shown that knowledge of dietary restrictions and knowledge of medical consequences of noncompliance have no effect on hemodialysis patient dietary compliance, and Durose et al noted that increased knowledge of phosphorus restrictions and medical complications actually had an inverse effect on phosphorus control.[19]
Further complicating this are food processing, packaging and labeling techniques and standards that may make it more difficult for patients to avoid high phosphorus containing foods. Many current food preservatives utilize high-phosphate ingredients, and may be compounding the problem of hyperphosphatemia.[27-29] We have previously shown that patients who adhere to a low sodium diet may inadvertently consume foods high in phosphorus, and visa versa.[30]
Our study suggests that despite dietary instruction, patients undergoing MD have a poor knowledge of dietary phosphorus content, compared with knowledge of other nutrients important in CKD. Interestingly, there was no difference in nutrition knowledge when comparing PD and HD patients, despite differences in education level and health literacy between the groups.
The study does have several limitations. First, there is no standardized test available in either the hemodialysis or peritoneal dialysis literature that has been validated to test nutrition knowledge. The test we utilized, the CKDKAT-N, was designed utilizing information taken directly from materials published by the National Kidney Foundation's KDOQI guidelines,[31] with nutrient analysis taken from the USDA Nutrient Database.[32] Content validity was assessed by 2 nephrologists, 1 nurse specializing in kidney disease, and 1 renal dietician. Other authors have published results of similar tests consisting of multiple choice questions or closed-ended questions to assess patient knowledge of nutrition.[8, 9, 19] In each case, content validity was similarly assessed by the medical practitioners and other providers, such as renal dieticians.
Second, our study was limited by its small size, consisting of dialysis patients with relatively homogeneous educational, cultural, and racial backgrounds. The homogeneity of education levels may have helped exclude that as a confounding variable, as patients were generally very well educated in our patient population. Nonetheless, this does limit the ability to generalize the results.
Finally, our study design did not allow for correlations between phosphorus knowledge and serum phosphorus values. While the intent of this analysis was to evaluate phosphorus knowledge with education and health literacy, as compared to knowledge of other nutrients, subsequent studies with larger populations and laboratory correlation may be useful to help elucidate the association of phosphorus knowledge with serum phosphorus levels, dietary intake and binder compliance. The mounting evidence that individual education, adequate health literacy, and dialysis modality do not translate into improved knowledge of dietary phosphorus suggests that the current standard of dietary consulting by nephrologists, dialysis nurses, and renal dieticians, coupled with judicious phosphate-binder prescription, may not be adequate to overcome this complex problem. More innovative methods for providing sustainable intensive counseling, newer phosphate-binders with longer durations of action, fewer side effects and improved affordability, or additional alternatives for low-phosphorus food items that also comply with the other restrictions in the CKD diet may ultimately be required.
Acknowledgments
The Authors would like to thank Dr. Arjang Djamali for assistance with reviewing the manuscript.
Funding: Dr. Jaffery is supported by NIH 5K12RR017614-03 and the Satellite Dialysis Clinical Scientist Award of the National Kidney Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Judson B. Pollock, Cincinnati, OH, USA.
Jonathan B. Jaffery, Assistant Professor of Medicine, University of Wisconsin, Madison, WI, USA.
References
- 1.Block GA, et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15(8):2208–18. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 2.Block GA, et al. Association of serum phosphorus and calcium × phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31(4):607–17. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 3.Ganesh SK, et al. Association of elevated serum PO(4), Ca × PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol. 2001;12(10):2131–8. doi: 10.1681/ASN.V12102131. [DOI] [PubMed] [Google Scholar]
- 4.Nishizawa Y, et al. Hyperphosphatemia and vascular calcification in end-stage renal disease. J Ren Nutr. 2005;15(1):178–82. doi: 10.1053/j.jrn.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 5.Raggi P, et al. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol. 2002;39(4):695–701. doi: 10.1016/s0735-1097(01)01781-8. [DOI] [PubMed] [Google Scholar]
- 6.Goldfarb S. Renal Osteodystrophy, Disorders of Divalent Ions, and Nephrolithiasis. Nephrology Self-Assessment Program, J Amer Soc Nephrol. 2005;4(5):245–279. [Google Scholar]
- 7.Cupisti A, et al. Dietary habits and counseling focused on phosphate intake in hemodialysis patients with hyperphosphatemia. J Ren Nutr. 2004;14(4):220–5. [PubMed] [Google Scholar]
- 8.Ford JC, et al. The effect of diet education on the laboratory values and knowledge of hemodialysis patients with hyperphosphatemia. J Ren Nutr. 2004;14(1):36–44. doi: 10.1053/j.jrn.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Schlatter S, Ferrans CE. Teaching program effects on high phosphorus levels in patients receiving hemodialysis. Anna J. 1998;25(1):31–6. discussion 37-8. [PubMed] [Google Scholar]
- 10.Kirsch IS, et al. Adult Literacy in America: A First Look at the Results of the National Adult Literacy Survey. U.S. Department of Education, NCES; Washington, DC: 1993. [Google Scholar]
- 11.Comings J, Reder S, Sum A. National Center for the Study of Adult Learning and Literacy; Cambridge, Mass.: 2001. Building a level playing field: the need to expand and improve thenational and state adult education and literacy systems. [Google Scholar]
- 12.Sum A, Kirsch IS, Taggart R. The Twin Challenges of Mediocrity and Inequality: Literacy in the U.S. from an International Perspective. Policy Information Center Educational Testing Service; Princeton, NJ: 2002. [Google Scholar]
- 13.Baker DW, et al. Functional health literacy and the risk of hospital admission among Medicare managed care enrollees. Am J Public Health. 2002;92(8):1278–83. doi: 10.2105/ajph.92.8.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baker DW, et al. Health literacy and the risk of hospital admission. J Gen Intern Med. 1998;13(12):791–8. doi: 10.1046/j.1525-1497.1998.00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gazmararian JA, et al. Health literacy among Medicare enrollees in a managed care organization. Jama. 1999;281(6):545–51. doi: 10.1001/jama.281.6.545. [DOI] [PubMed] [Google Scholar]
- 16.Schillinger D, et al. Association of health literacy with diabetes outcomes. Jama. 2002;288(4):475–82. doi: 10.1001/jama.288.4.475. [DOI] [PubMed] [Google Scholar]
- 17.Williams MV, et al. Inadequate functional health literacy among patients at two public hospitals. Jama. 1995;274(21):1677–82. [PubMed] [Google Scholar]
- 18.Jain S, et al. Health Literacy Skills of Chronic Hemodialysis Patients; American Society of Nephrology Annual Meeting; Philadelphia. 2005. Abstract. [Google Scholar]
- 19.Durose CL, et al. Knowledge of dietary restrictions and the medical consequences of noncompliance by patients on hemodialysis are not predictive of dietary compliance. J Am Diet Assoc. 2004;104(1):35–41. doi: 10.1016/j.jada.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 20.Guideline 6: Dietary and Other Therapeutic Lifestyle Changes in Adults. American Journal of Kidney Diseases. 2004;43(5):s115–119. [Google Scholar]
- 21.National Kidney Foundation Website. KDOQI Clinical Practice Guidelines. accessed 27 June 2005 [cited 27 June 2005] [Google Scholar]
- 22.United States Department of Agriculture Nutrient Data Laboratory National Nutrient Database. accessed: 6 Jan 2006 [cited 6 Jan 2006] [Google Scholar]
- 23.Baker DW, et al. Development of a brief test to measure functional health literacy. Patient Educ Couns. 1999;38(1):33–42. doi: 10.1016/s0738-3991(98)00116-5. [DOI] [PubMed] [Google Scholar]
- 24.Gazmararian JA, et al. Health literacy and knowledge of chronic disease. Patient Educ Couns. 2003;51(3):267–75. doi: 10.1016/s0738-3991(02)00239-2. [DOI] [PubMed] [Google Scholar]
- 25.Slinin Y, Foley RN, Collins AJ. Calcium, phosphorus, parathyroid hormone, and cardiovascular disease in hemodialysis patients: the USRDS waves 1, 3, and 4 study. J Am Soc Nephrol. 2005;16(6):1788–93. doi: 10.1681/ASN.2004040275. [DOI] [PubMed] [Google Scholar]
- 26.Qunibi WY, et al. Treatment of hyperphosphatemia in hemodialysis patients: The Calcium Acetate Renagel Evaluation (CARE Study) Kidney Int. 2004;65(5):1914–26. doi: 10.1111/j.1523-1755.2004.00590.x. [DOI] [PubMed] [Google Scholar]
- 27.Karalis M, Murphy-Gutekunst L. Patient education. Enhanced foods: hidden phosphorus and sodium in foods commonly eaten. J Ren Nutr. 2006;16(1):79–81. doi: 10.1053/j.jrn.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Murphy-Gutekunst L. Hidden phosphorus in popular beverages. Nephrol Nurs J. 2005;32(4):443–5. [PubMed] [Google Scholar]
- 29.Uribarri J, Calvo MS. Hidden sources of phosphorus in the typical American diet: does it matter in nephrology? Semin Dial. 2003;16(3):186–8. doi: 10.1046/j.1525-139x.2003.16037.x. [DOI] [PubMed] [Google Scholar]
- 30.Jaffery JB, Hood VL. Conflicting dietary advice for adhering to low-sodium and low-phosphorus diets. J Ren Nutr. 2006;16(4):332–6. doi: 10.1053/j.jrn.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 31.KDOQI Clinical Practice Guidelines. National Kidney Foundation; 2006. [Google Scholar]
- 32.United States Department of Agriculture Nutrient Data Laboratory National Nutrient Database.