Abstract
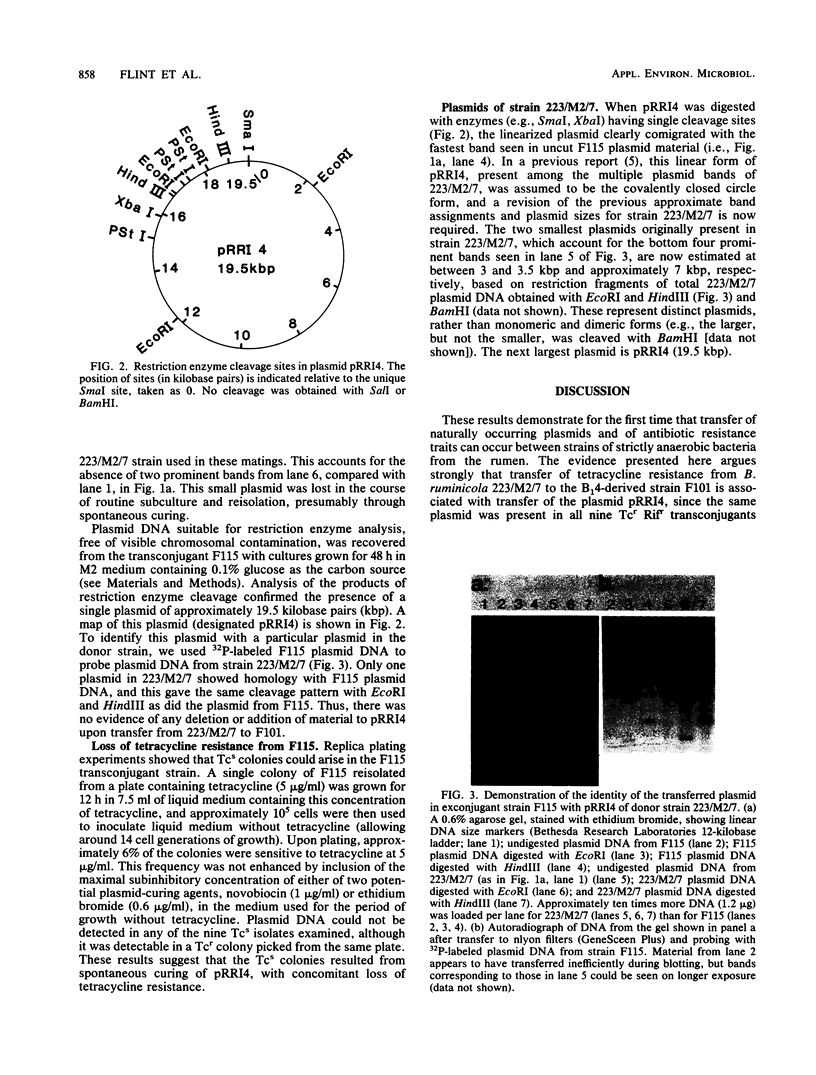
Tetracycline resistance was transferred at frequencies between 10(-7) and 10(-6) per recipient cell in anaerobic matings between two strains of the strictly anaerobic rumen bacterium Bacteroides ruminicola. The donor strain, 223/M2/7, was a multiple-plasmid-bearing tetracycline-resistant strain from the ovine rumen, and the recipient, F101, was a rifampin-resistant mutant of B14, a bovine strain belonging to B. ruminicola subsp. brevis. Resistance transfer could occur in the presence of DNase, but not in dummy mating mixtures in which filtrate from a donor culture replaced donor cells. Acquisition of tetracycline resistance by the recipient was accompanied by the appearance of a 19.5-kilobase pair plasmid (designated pRRI4) which was homologous with a plasmid of similar size and restriction pattern present in the donor strain. A transconjugant (F115) carrying pRRI4 was also able to act as a donor of tetracycline resistance and plasmid DNA in matings with another recipient. Derivatives of F115 that had spontaneously lost tetracycline resistance lacked detectable plasmid DNA. It is concluded that pRRI4 mediated the transfer of tetracycline resistance. Transfer of resistance was not detectably enhanced by pregrowth of the donor in medium containing tetracycline. Transfer of tetracycline resistance was not detected from 223/M2/7 to a strain, 23 belonging to B. ruminicola subsp. ruminicola.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRYANT M. P., SMALL N., BOUMA C., CHU H. Bacteroides ruminicola n. sp. and Succinimonas amylolytica; the new genus and species; species of succinic acid-producing anaerobic bacteria of the bovine rumen. J Bacteriol. 1958 Jul;76(1):15–23. doi: 10.1128/jb.76.1.15-23.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin R. L., Allison M. J. Rumen metabolism. J Anim Sci. 1983 Jul;57 (Suppl 2):461–477. [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- Guiney D. G., Jr, Hasegawa P., Davis C. E. Expression in Escherichia coli of cryptic tetracycline resistance genes from bacteroides R plasmids. Plasmid. 1984 May;11(3):248–252. doi: 10.1016/0147-619x(84)90031-3. [DOI] [PubMed] [Google Scholar]
- Martínez-Suárez J. V., Baquero F., Reig M., Pérez-Díaz J. C. Transferable plasmid-linked chloramphenicol acetyltransferase conferring high-level resistance in Bacteroides uniformis. Antimicrob Agents Chemother. 1985 Jul;28(1):113–117. doi: 10.1128/aac.28.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays T. D., Smith C. J., Welch R. A., Delfini C., Macrina F. L. Novel antibiotic resistance transfer in Bacteroides. Antimicrob Agents Chemother. 1982 Jan;21(1):110–118. doi: 10.1128/aac.21.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privitera G., Sebald M., Fayolle F. Common regulatory mechanism of expression and conjugative ability of a tetracycline resistance plasmid in Bacteroides fragilis. Nature. 1979 Apr 12;278(5705):657–659. doi: 10.1038/278657a0. [DOI] [PubMed] [Google Scholar]
- Russell J. B. Fermentation of cellodextrins by cellulolytic and noncellulolytic rumen bacteria. Appl Environ Microbiol. 1985 Mar;49(3):572–576. doi: 10.1128/aem.49.3.572-576.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., Shoemaker N. B., Guthrie E. P. Recent advances in Bacteroides genetics. Crit Rev Microbiol. 1987;14(1):49–71. doi: 10.3109/10408418709104435. [DOI] [PubMed] [Google Scholar]
- Shah H. N., Collins M. D. Genus Bacteroides. A chemotaxonomical perspective. J Appl Bacteriol. 1983 Dec;55(3):403–416. doi: 10.1111/j.1365-2672.1983.tb01680.x. [DOI] [PubMed] [Google Scholar]
- Shoemaker N. B., Getty C., Guthrie E. P., Salyers A. A. Regions in Bacteroides plasmids pBFTM10 and pB8-51 that allow Escherichia coli-Bacteroides shuttle vectors to be mobilized by IncP plasmids and by a conjugative Bacteroides tetracycline resistance element. J Bacteriol. 1986 Jun;166(3):959–965. doi: 10.1128/jb.166.3.959-965.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tally F. P., Snydman D. R., Shimell M. J., Malamy M. H. Characterization of pBFTM10, a clindamycin-erythromycin resistance transfer factor from Bacteroides fragilis. J Bacteriol. 1982 Aug;151(2):686–691. doi: 10.1128/jb.151.2.686-691.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A., Jones K. R., Macrina F. L. Transferable lincosamide-macrolide resistance in Bacteroides. Plasmid. 1979 Apr;2(2):261–268. doi: 10.1016/0147-619x(79)90044-1. [DOI] [PubMed] [Google Scholar]