Abstract
The effects of increased unsaturation in the sn-2 fatty acyl chain of phosphatidylcholines (PCs) on the lipid lateral diffusion have been investigated by pulsed-field gradient NMR. Macroscopically oriented bilayers containing a monosaturated PC, egg sphingomyelin, and cholesterol (CHOL) have been studied at temperatures between 0°C and 60°C, and the number of double bonds in the PC was one, two, four, or six. For PC bilayers, with and without the incorporation of egg sphingomyelin and CHOL, the lateral diffusion increased with increasing number of double bonds, as a consequence of the increased headgroup area caused by the unsaturation. Addition of CHOL caused a decrease in lipid diffusion due to the condensing effect of CHOL on the headgroup area. Phase separation into large domains of liquid-disordered and liquid-ordered phases were observed in the ternary systems with PCs containing four and six double bonds, as evidenced by the occurrence of two lipid diffusion coefficients. PC bilayers with one or two double bonds appear homogeneous on the length scales probed by the experiment, but the temperature dependence of the diffusion suggests that small domains may be present also in these ternary systems.
INTRODUCTION
Polyunsaturated fatty acids (PUFA) are essential substances for humans and other higher animals. Lipids containing PUFA are found in very high proportions in neural tissue, and they are important for the development and function of the brain and visual system (1). Apart from these very specialized tissues, in which PUFA may reach 50% of the total fatty acyl chains, PUFA lipids are also found in smaller amounts in other tissues. The levels of PUFA in these membranes can be altered by dietary constraints, and an intake of these fatty acids are considered beneficial to the health. The most studied PUFA, docosohexaenoic acid (DHA), is positively linked to the prevention of an enormous variety of human afflictions, including cancer, heart disease, rheumatoid arthritis, asthma, lupus, alcoholism, and many others (2). For one simple molecule to be able to affect so many seemingly unrelated processes, it seems plausible that its function is not specific but rather that it acts at a fundamental level, common to most cells. This fundamental level, most probably, is connected with the physical properties of the cell membranes, where lipids with PUFA are functioning in the body.
It is well known that the packing of lipids, and therefore their shape, is a very important property for a functioning membrane. This property determines the curvature and elasticity of the membrane. It was shown more than two decades ago that the lipid composition of so-called lamellar- and nonlamellar-forming lipids in the membranes of the bacteria Acholeplasma laidlawii and Escherichia coli was regulated to keep an optimal packing or spontaneous curvature for the proteins in the cell membrane (3,4). Similarly, as shown by Brown and co-workers, there is a balance between bilayer-forming and nonbilayer-forming lipids in the native retinal rod membranes, and here the lipids are polyunsaturated with a high content of DHA chains (5). These authors showed that the polyunsaturation has a strong influence on the rhodopsin function, and the molecular packing of wedge-shaped, polyunsaturated lipids was elegantly developed into their “flexible surface model”.
PUFA is primarily found in the sn-2 chain in phosphatidylethanolamine (PE), phosphatidylcholine (PC), and phosphatidylserine (PS), with the sn-1 chain being mainly a saturated fatty acid, like palmitic or stearic acid (6). A picture of the way PUFA affects the membrane structure and dynamics is emerging through recent studies of such molecules. Experimental and simulation studies have shown that the DHA chain is extremely flexible, characterized by a high degree of molecular disorder and a rapid interconversion among a diverse set of conformational states (7–9). This is due to a reduced energy barrier for rotational isomerization about the single-C-C bonds that separate the unsaturated carbon atoms in PUFA. Because of this flexibility the DHA chain can rearrange its position in the monolayer to a larger extent than a saturated chain, and both experimental and simulated data show that part of the DHA can even reach up into the polar/apolar interface of the membrane (7,10,11). Also the chain ordering of the adjacent sn-1 saturated chain is lowered by the presence of the PUFA in the sn-2 chain, and the overall properties of membranes, including chain-melting transition temperature, lipid dynamics, phase behavior, elastic compressibility, permeability, fusion, and flip-flop, are influenced by the introduction of PUFA (2). The effects are largest for the first and second double bond and then rapidly level off for the introduction of three and more double bonds (2,12).
Another interesting property of polyunsaturated lipids is their possible role in membrane domain formation. There have been a few examples of DHA-induced lipid phase separations in lipid bilayers, in which both liquid-solid and liquid-liquid phase equilibria have been proposed (13). Since cholesterol (CHOL) is believed to be important for the formation of the so-called liquid-ordered (lo) phase (14,15), several studies have been aimed at the interaction of CHOL with lipids containing PUFA. A general conclusion from these studies is that CHOL interacts only weakly with polyunsaturated chains. This leads to a low solubility of CHOL in dipolyunsaturated lipids, e.g., for di-20:4PC and di-22:6PC it is found to be on the order of 10–15 mol %, whereas CHOL solubility in 18:0–20:4PC and 18:0–22:6PC is on the order of 50%–55% mol % (16). Consequently, the effect of CHOL on membrane properties levels off at low amounts of CHOL for diunsaturated lipids as compared to monosaturated or disaturated lipids (17). The CHOL molecule is also characterized by a larger tilt angle and smaller molecular order in the diunsaturated lipids (16).
A combined 2H and 1H magic angle spinning NMR study showed that CHOL interacts more favorably with the saturated chain in 18:0-X lipids in which X denotes chains of variable unsaturation (9). This leads to the fact that CHOL is most able to order the sn-1 chain and also that the ordering of this chain decreases as the unsaturation of the sn-2 chain increases. Concomitantly, a smaller CHOL-induced area condensation was also observed for all the polyunsaturated phospholipids compared to the monounsaturated PCs.
A molecular dynamics simulation study (11) suggests that the effect of CHOL on the phospholipid acyl chains is highly nonuniform in that the saturated chain shows only a negligible response (essentially a slight increase in the projected length along the bilayer normal), whereas the DHA chain undergoes a significant redistribution of its chain segments from the interfacial region to the hydrophobic core of the bilayer. Thus, a DHA chain can adopt a diverse set of chain conformations with similar (low) energies, whereas a stearic acid has a comparatively reduced conformational freedom.
To our knowledge no direct determination of the lateral diffusion of any polyunsaturated lipids with or without CHOL has so far been published. Polyunsaturated membranes have been proposed to be highly fluid (2), implying a fast lipid translational motion. However, other factors such as acyl chain entanglement and lipid free area will also affect the lateral diffusion in the bilayer. The pulsed-field gradient (pfg) NMR method can be used to measure the lipid lateral diffusion coefficients (DL) in macroscopically oriented bilayers (18) and, in an attempt to systematically study the effect on DL of lipid packing and domain formation, several systems containing various lipids and CHOL have been investigated (19–24). These studies are now extended to include PCs with an 18:0 chain in the sn-1 position and with an 18:1, 18:2, 20:4, or 22:6 sn-2 chain.
MATERIALS AND METHODS
Materials
The following substances were used for the preparation of macroscopically aligned lipid bilayers (Fig. 1): 1-stearoyl-2-oleoyl-sn-glycero-3-PC (SOPC), 1-stearoyl-2-linoleoyl-sn-glycero-3-PC (SLPC), 1-stearoyl-2-arachidonoyl-sn-glycero-3-PC (SAPC), 1-stearoyl-2-docosahexaenoyl-sn-glycero-3-PC (SDPC), and egg sphingomyelin (eSM) were obtained from Avanti Polar Lipids (Alabaster, AL) as solutions in chloroform. CHOL and deuterated water (2H2O) were purchased from Sigma (St. Louis, MO).
FIGURE 1.
Structures for the lipids used in this study.
Preparation of macroscopically oriented bilayers
Macroscopically oriented bilayers were prepared after a procedure reported earlier (18). Appropriate amounts of lipids were dissolved in a mixture of methanol and propanol (1:4 vol) at a concentration of 15 mg/ml. The solution was deposited on glass plates, the solvent was evaporated, and the plates were placed under high vacuum overnight to remove traces of solvent. The plates were then stacked and placed in a glass tube with a square cross section (∼30 plates/sample). The sample tube was placed for several days in a humid atmosphere at room temperature. During this time, hydrated and oriented bilayers were formed. After addition of water in excess, the tube was sealed and the sample was left for several hours for final equilibration. Sample orientation was checked with crossed polarizers. Because of the high sensitivity of polyunsaturated lipids to oxidation, all handling of the lipids was performed in a dry nitrogen gas atmosphere. Water used for hydration was depleted of dissolved oxygen by nitrogen gas bubbling.
Pulsed-field gradient NMR technique
The diffusion measurements were performed on a Chemagnetics Infinity (Varian, Fort Collins, CO) NMR spectrometer operating at a proton frequency of 100 MHz and equipped with a specifically designed goniometer probe that enabled macroscopically aligned bilayers to be oriented with the bilayer normal at the magic angle (54.7°) with respect to the main magnetic field. This causes the dipolar interactions to vanish, resulting in a significant reduction of the line width. Details of the pfg-NMR method for measurements of lipid lateral diffusion on macroscopically oriented bilayers can be found elsewhere (18). For all measurements the stimulated spin-echo pulse sequence was used (25). The diffusion decay of the echo amplitude, A, can be described by the equation
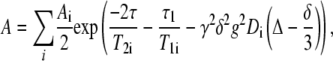 |
(1) |
where the summation index goes over all diffusion components present, T1 and T2 are the longitudinal and transverse proton NMR relaxation times, γ is the gyromagnetic ratio, Δ is the time interval between two identical gradient pulses (equal to the diffusion time), δ and g are the duration and amplitude of the pfgs, respectively, and D is the self-diffusion coefficient. τ and τ1 are times in the pulse sequence that govern the T2 and T1 relaxation intervals.
In our experiments g = 1.15T/m and δ was varied in the range 1–9 ms in 10–32 steps and all other variables were kept constant. The ranges of τ and τ1 were 11 and 50–200 ms, respectively. The resulting D was not dependent on the choice of these parameters. Signal accumulations from 32 to 160 were made to obtain an acceptable signal/noise level. Experimental data were Fourier transformed into a set of spectra that was analyzed with the component resolved method to extract the diffusion coefficient. This method globally fits all frequency channels and results in spectral shapes and diffusion coefficients for the individual components of the data (26). The lateral diffusion coefficient DL was calculated as 1.5D due to the angle between the pfg and the main magnetic field (18).
The observation of domain formation is based on the difference in DL and the apparent activation energy for the diffusion process in the ld and lo phases (27). If the domains are large enough to make the lifetime within the domains long compared to the diffusion time, two separate DLs will be observed. The size of the domains typically needs to be larger than 1 μm for this to occur. If the domains are smaller than this, a weighted average of the DLs in the two phases will be observed.
RESULTS AND DISCUSSION
PC and PC/CHOL bilayers
The diffusion decays for the lipid systems containing either PC or PC/CHOL were biexponential; the faster component corresponds to water. This component has not been analyzed further. The slow component, corresponding to lipids, gave NMR spectra with a characteristic appearance of PCs with the most prominent peaks at 0.9 ppm (ω-CH3), 1.1 ppm (chain -CH2-), and 3.1 ppm (choline-CH3). For higher degrees of unsaturation, a signal from the -CH=CH- protons could also be seen at 5.2 ppm. (The chemical shifts are only approximate but are in good agreement with MAS NMR spectra of saturated and polyunsaturated chains (7,28).) Spectra for samples with varying CHOL content are shown in Fig. 2. No signal from CHOL could be observed, because of the fast transverse NMR relaxation of the CHOL protons. Note the larger decrease in signal intensity for the peaks arising from the lipid chains as the CHOL content increases. This is an effect of the ordering of the chains caused by CHOL, increasing the T2 relaxation rate.
FIGURE 2.
1H-NMR spectra at 21°C from oriented samples of the binary systems PC/CHOL, obtained from the pfg-NMR experiments after water suppression. (Solid lines) 0% CHOL, (dashed lines) 4% CHOL, (dash-dotted lines) 40% CHOL. (A) SOPC, (B) SLPC, (C) SAPC, (D) SDPC.
The influences of the CHOL concentration and temperature on DL are shown in Fig. 3. An increase in unsaturation from SOPC to SDPC leads to an increase in DL at all CHOL concentrations, and an increase in the CHOL content results in a decrease in DL for all phospholipids. DL increases with temperature for all the PCs.
FIGURE 3.
Lateral diffusion coefficients as a function of CHOL content for the temperatures 21°C (circles), 30°C (triangles down), 40°C (squares), 50°C (diamonds), and 60°C (triangles up) for the binary systems of PC/CHOL. (A) SOPC, (B) SLPC, (C) SAPC, (D) SDPC.
The effect of unsaturation can be rationalized if the effect of packing of the lipids on DL is considered. Vaz et al. (29) and Almeida et al. (30) showed that a free area theory of Cohen and Turnbull (31) could be used to describe the diffusion process in lipid bilayers. This theory considers a particle performing a random walk in two dimensions. Each elementary step in this process is limited by the occurrence of a free volume (or free area af) greater than a critical size (a*) next to the diffusing particle. This leads to the following relation between the lateral diffusion coefficient and the free area (32):
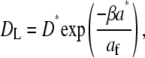 |
(2) |
where D* is a constant, and β is a factor to correct for overlapping free volumes (typically in the range 0.5–1). The free area can be estimated as  where aav is the average area for the molecule in the bilayer. The values of aav for monolayers (12) and bilayers (33) are quite similar, and monolayer data are used here. When data are fit to Eq. 2, values for a* between 43 and 48 Å2 are found depending on the chosen value of β. Fig. 4 shows the results using β = 1. a* is usually taken to be the van der Waals, area and the value of ∼40 Å2 seems reasonable for the PC molecules. For example, the area per molecule obtained in the limit of high CHOL content and high lateral pressure in monolayers at 25°C is found to be close to 40 Å2 for all lipids (12). The good fit to Eq. 2 and the reasonable magnitude for the fitting parameters show that the increase in DL with increasing unsaturation for our systems is consistent with the increasing area per molecule with increasing unsaturation seen in monolayers (12) and bilayers (33).
where aav is the average area for the molecule in the bilayer. The values of aav for monolayers (12) and bilayers (33) are quite similar, and monolayer data are used here. When data are fit to Eq. 2, values for a* between 43 and 48 Å2 are found depending on the chosen value of β. Fig. 4 shows the results using β = 1. a* is usually taken to be the van der Waals, area and the value of ∼40 Å2 seems reasonable for the PC molecules. For example, the area per molecule obtained in the limit of high CHOL content and high lateral pressure in monolayers at 25°C is found to be close to 40 Å2 for all lipids (12). The good fit to Eq. 2 and the reasonable magnitude for the fitting parameters show that the increase in DL with increasing unsaturation for our systems is consistent with the increasing area per molecule with increasing unsaturation seen in monolayers (12) and bilayers (33).
FIGURE 4.
Lateral diffusion versus free area for the PC bilayers. The line is the best fit to Eq. 2 with β set to 1. The corresponding values of D* and a* are 53 μm2/s and 43 Å2, respectively.
It can be inferred from Fig. 3 that the dependence of DL on the CHOL concentration is approximately linear for SOPC, SLPC, and SAPC. Such a linear dependence has been observed for binary systems with 1-palmitoyl-2-oleoyl-PC and dioleoyl-PC with CHOL (19), which are generally believed to form only homogeneous phases. For SDPC the dependence is more complicated. For CHOL contents smaller than 10% and larger than 25%, DL varies very little, whereas in the interval 10%–25%, DL has quite a strong linear dependence on the CHOL content. Such a behavior was also observed in the binary system of eSM/CHOL (19), where it was interpreted as evidence of the formation of microdomains. However, in this system it is difficult to conclude that lo domains are formed, since the activation energies more closely conform to those usually found in the ld phase (vide infra). At low CHOL contents the effect is complicated by the fact that there may be two opposing events. In general, the condensing effect of CHOL will decrease the lipid-free volume in the bilayer, causing a decrease in DL. However, small additions of CHOL can also result in an increase in DL caused by the reduced entanglement of the lipid chains (34,35). These two effects might balance out to give a constant DL at low CHOL concentrations. The reason for the constant value of DL at high CHOL concentrations is not clear. It seems improbable that the reason is poor solubility of CHOL, since the solubility limit in SDPC bilayers is reported to be 55% (36). More studies are needed to get an understanding of this feature.
The temperature dependence of DL gives a straight line in an Arrhenius plot, from which an apparent activation energy (EA) for the diffusion process can be calculated. Fig. 5 shows the influence of the CHOL concentration on EA. It is found that an increase in lipid unsaturation generally results in a decrease in EA. This was also observed in an earlier study (19). Moreover, up to a CHOL content of 10 mol % EA is almost constant, whereas between 10–40 mol % of CHOL EA increases approximately linearly. However, the increase is rather moderate and the values of EA are all in the range of what is obtained for other systems in the ld phase (19,20). Taken together, the data for the binary systems indicate that the bilayers are in a homogeneous ld phase, although we cannot exclude the existence of small amounts of lo phase.
FIGURE 5.
Apparent activation energies for the diffusion process in the binary systems of PC/CHOL as a function of the CHOL content.
PC/eSM/CHOL bilayers
The compositions of the bilayers were 37.5/37.5/25 mol % of PC/eSM/CHOL. The temperature dependence of the diffusion coefficients for the ternary systems are shown as circles in Fig. 6 and as Arrhenius plots in Fig. 7. For comparison, data from the binary systems are included in Fig. 6 as solid and dashed lines. Only a single lipid diffusion coefficient is observed in the SOPC and SLPC ternary systems (Figs. 6 and 7, A and B) with DLs intermediate between those observed for the binary systems of eSM/CHOL and PC/CHOL. This “averaging” of the diffusion coefficients in mixed bilayers has been observed previously in other systems (23,37,38) and reflects the fact that the lateral diffusion is governed by the physicochemical properties of the bilayer as a whole and not by the properties of the individual lipids. The bilayers are thus homogeneous in these two systems at all temperatures on the timescale of the diffusion experiment (50–200 ms), i.e., possible domains must be smaller than ∼1 μm.
FIGURE 6.
Lateral diffusion coefficients as a function of temperature for the ternary systems of PC/eSM/CHOL. The circles indicate diffusion in the ternary systems, and the lines are taken from the binary systems for comparison. (Solid lines) PC (upper) and eSM (lower) diffusion in the pure lipid systems. (Dashed lines) PC (upper) and eSM (lower) diffusion interpolated to a CHOL content of 25% for the binary systems of PC/CHOL and eSM/CHOL, respectively. The values for the eSM systems are taken from Filippov et al. (19).
FIGURE 7.
Temperature dependence of DL obtained for the ternary systems of PC/eSM/CHOL. The lines are best fits to the Arrhenius equation and give the following apparent activation energies: (A) SOPC: EA = 49 kJ/mol, (B) SLPC: EA = 49 kJ/mol, (C) SAPC: EA = 24 (ld) and 68 (lo) kJ/mol (4), (D) SDPC: EA = 29 (ld) and 78 (lo) kJ/mol.
The ternary systems with SAPC and SDPC show a different behavior (Figs. 6 and 7, C and D), and the diffusion decay for the lipids can only be sufficiently described by a biexponential decay, giving two separate diffusion coefficients. According to observations in similar systems (20,21,24,27), we interpret this finding as a result of a phase separation into the ld and lo phases. DL is ∼2–10 times slower in the lo phase, with the largest difference at low temperatures.
It is difficult to determine the composition of the two phases from the diffusion data, since the signals from the phospholipids overlap and no signal is observed from CHOL. Furthermore, since the relaxation rates differ in the two phases, no direct measure of the relative amounts of the phases can be made (see Eq. 1). However, it is possible to make an estimate of the composition of the two phases from the magnitude of the observed DLs and the appearance of the spectra of the two components. We assume that the lo phase is enriched in the saturated lipid, whereas the ld phase is enriched in the unsaturated lipid. This is in accordance with phase diagrams in similar systems (23,39,40). Thus, if it is assumed as a first approximation that all eSM goes into the lo phase and all PC goes into the ld phase, it is possible to estimate the CHOL content in the phases by comparison with the binary systems. If we compare DL for the ld phase with data from the binary PC/CHOL systems, we find that it corresponds to a CHOL content of 30%–40%. This gives a CHOL content in the lo phase of 20% or less. The corresponding comparison of DL for the lo phase to those in the eSM/CHOL system indicates that very little CHOL is located in the lo phase. This finding is rather surprising since CHOL generally is found to be slightly enriched in the lo phase.
A plausible explanation is that small amounts of eSM are found in the ld phase and some of the unsaturated PC goes into the lo phase. Thus, the diffusion would be slowed down in the ld phase and speeded up in the lo phase and a direct comparison with the binary systems will overestimate the amount of CHOL in the ld phase. If we look at the spectra obtained for the lo phase (Fig. 8, C and D), no peak from the double bond region at 5.2 ppm is seen. This is an indication that the amount of PC in this phase is low, since this peak is visible in the binary spectra for CHOL concentrations up to 40% at this temperature. Furthermore, a comparison of the spectrum for the ld phase with those found for the binary PC/CHOL systems reveals that it is similar in appearance as that for 20%–30% CHOL. Thus, the amount of CHOL is substantial in the ld phase, and CHOL seems to partition fairly equally into the two phases, with only a smaller preference for the lo phase. This proposal is in agreement with tie-lines found in dioleoyl-PC/dipalmitoyl-PC/CHOL systems (40), whereas other investigations indicate a more distinct partition of CHOL into the lo phase (41,42). To further investigate the partitioning issues, we are currently planning measurements with deuterated palmitoyl-SM as well as deuterated CHOL.
FIGURE 8.
Spectra obtained from the component resolved analysis of the pfg-NMR diffusion experiments at 40°C for the ternary systems of PC/eSM/CHOL.
Further insight into the nature of the two phases is given by the temperature dependence of DL. Previous studies have shown that EA is significantly larger for the lo phase compared to the ld phase (19,20), making it possible to discriminate between the two phases. For the SAPC and SDPC systems EA is found to be 24 and 29 kJ/mol, respectively, for the ld phase, whereas it is much higher (68 and 78 kJ/mol) for the lo phase (Fig. 7). For the SOPC and SLPC systems EA is found to be intermediate between these two extremes: 49 kJ/mol for both systems. This may indicate that these systems also form the lo phase but that the domains formed are small enough to allow for a lipid exchange between the two phases that is fast on the timescale considered for the diffusion experiment. Then, the observed DL will be an average of the lateral diffusion in the two phases and, provided that the amounts of the two phases do not change significantly with temperature, EA will also be averaged.
Domain formation in ternary systems
A large number of ternary systems has been investigated with regard to domain formation and they all have common features, i.e., they contain one saturated and one unsaturated lipid, together with a sterol. Systematic pfg-NMR studies have revealed that each of these three components are essential for domain formation and that small changes in the structure of the components can have a large impact on the domain-forming process (20,21,23,24). It has been found that the homogeneity of the SM compound was of crucial importance for the domain formation and that small changes in the structure of the sterol also strongly could affect the process. It was therefore interesting to see how the degree of unsaturation influences the lateral phase separation. Domain formation has been observed for the diunsaturated DOPC with eSM and CHOL, whereas for the monounsaturated POPC it is less clear whether large domains are formed or not.
There are large differences in the two phase diagrams that have been reported (39,41), and pfg-NMR methods have been unable to detect domains in this system (G. Orädd and G. Lindblom, unpublished results). This discrepancy probably originates from differences in spatial resolution of the methods. Fluorescence microscopy and pfg-NMR rely on large (>μm) domains, whereas fluorescent anisotropy measurements can detect smaller domains. For the similar systems SOPC/eSM/CHOL and SLPC/eSM/CHOL, we find no evidence for large domains, although the temperature dependence of DL in these systems could be taken as evidence for the existence of small domains. However, increasing the degree of unsaturation in the lipid chains results in phase separation into the ld and lo phases for both the SAPC/eSM/CHOL and the SDPC/eSM/CHOL systems. Therefore, systems that are on the verge of forming larger domains can be triggered into this behavior by an increase in the number of double bonds in monounsaturated PCs.
CONCLUSIONS
We have investigated the effect of increased unsaturation in the fatty acyl chain of PCs on the lateral diffusion in bilayers containing a monosaturated PC, eSM, and CHOL. It can be concluded that an increase in the number of double bonds in the sn-2 chain of the monosaturated PC results in an increase in the free area of the bilayers. For the one-component PC bilayers, this leads to an increase in the lateral diffusion in accordance with theoretical predictions (Eq. 2). It also influences other bilayer properties, such as the lateral compressibility, bending rigidity, and lipid chain localization. Addition of CHOL to such systems will have an ordering effect, primarily on the saturated chain but also on the unsaturated chain. As a consequence of this, the free area in the bilayers will decrease, resulting in a decreased lateral diffusion. In the ternary systems of PC/eSM/CHOL the ordering by CHOL on eSM will be more effective than on PC, and a lateral phase separation into ld and lo phases is observed for the most unsaturated PCs. In these systems the lo phase contains mostly eSM, whereas the ld phase is enriched in the unsaturated PC. We propose that the phase separation is driven by the increasing difficulty of incorporating an unsaturated lipid into the highly ordered matrix formed by eSM and CHOL.
Acknowledgments
This work was supported by the Swedish Research Council and the Knut and Alice Wallenberg Foundation. A.F. acknowledges support from grant CRDF REC-007-3 RNP.2.1.1.3222.
Andrey Filippov's permanent address is Dept. Molecular Physics, Kazan State University, 420008 Kazan, Russia.
Editor: Lukas K. Tamm.
References
- 1.Mouritsen, O. G. 2005. Life - As a Matter of Fat: The Emerging Science of Lipidomics. Springer, Heidelberg.
- 2.Stillwell, W., and S. R. Wassall. 2003. Docosahexaenoic acid: membrane properties of a unique fatty acid. Chem. Phys. Lipids. 126:1–27. [DOI] [PubMed] [Google Scholar]
- 3.Lindblom, G., I. Brentel, M. Sjölund, G. Wikander, and Å. Wieslander. 1986. Phase equilibria of membrane lipids from Acholeplasma laidlawii. The importance of a single lipid forming nonlamellar phases. Biochemistry. 25:7502–7510. [DOI] [PubMed] [Google Scholar]
- 4.Rilfors, L., and G. Lindblom. 2002. Regulation of lipid composition in biological membranes—biophysical studies of lipids and lipid synthesizing enzymes. Colloids Surf. B. 26:112–124. [Google Scholar]
- 5.Brown, M. F. 1994. Modulation of rhodopsin function by properties of the membrane bilayer. Chem. Phys. Lipids. 73:159–180. [DOI] [PubMed] [Google Scholar]
- 6.Anderson, R. E., and L. Sperling. 1971. Lipids of ocular tissues. VII. Positional distribution of the fatty acids in the phospholipids of bovine retina rod outer segments. Arch. Biochem. Biophys. 144:673–677. [DOI] [PubMed] [Google Scholar]
- 7.Eldho, N. V., S. E. Feller, S. Tristram-Nagle, I. Polozov, and K. Gawrisch. 2003. Polyunsaturated docosahexaenoic vs docosapentaenoic acid—differences in lipid matrix properties from the loss of one double bond. J. Am. Chem. Soc. 126:6409–6421. [DOI] [PubMed] [Google Scholar]
- 8.Feller, S. E., K. Gawrisch, and A. D. MacKerrell. 2001. Polyunsaturated fatty acids in lipid bilayers: intrinsic and environmental contributions to their unique physical properties. J. Am. Chem. Soc. 124:318–326. [DOI] [PubMed] [Google Scholar]
- 9.Huster, D., K. Arnold, and K. Gawrisch. 1998. Influence of docosahexaenoic acid and cholesterol on lateral lipid organization in phospholipid mixtures. Biochemistry. 37:17299–17308. [DOI] [PubMed] [Google Scholar]
- 10.Everts, S., and J. H. Davis. 2000. H-1 and C-13 NMR of multilamellar dispersions of polyunsaturated (22: 6) phospholipids. Biophys. J. 79:885–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pitman, M. C., F. Suits, A. D. MacKerrell, and S. E. Feller. 2004. Molecular-level organization of saturated and polyunsaturated fatty acids in a phosphatidylcholine bilayer containing cholesterol. Biochemistry. 43:15318–15328. [DOI] [PubMed] [Google Scholar]
- 12.Smaby, J. M., M. M. Momsen, H. L. Brockman, and R. E. Brown. 1997. Phosphatidylcholine acyl unsaturation modulates the decrease in interfacial elasticity by cholesterol. Biophys. J. 73:1492–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stillwell, W., L. J. Jenski, M. Zerouga, and A. C. Dumaual. 2000. Detection of lipid domains in docosahexaenoic acid-rich bilayers by acyl chain-specific FRET probes. Chem. Phys. Lipids. 104:113–132. [DOI] [PubMed] [Google Scholar]
- 14.Ipsen, J. H., G. Karlström, O. G. Mouritsen, H. W. Wennerström, and M. J. Zuckermann. 1987. Phase equilibria in the phosphatidylcholine-cholesterol system. Biochim. Biophys. Acta. 905:162–172. [DOI] [PubMed] [Google Scholar]
- 15.Vist, M. R., and J. H. Davis. 1990. Phase equilibria of cholesterol/ dipalmitoylphosphatidylcholine mixtures: 2H nuclear magnetic resonance and differential scanning calorimetry. Biochemistry. 29:451–464. [DOI] [PubMed] [Google Scholar]
- 16.Brzustowicz, M. R., V. Cherezov, M. Caffrey, W. Stillwell, and S. R. Wassall. 2002. Molecular organization of cholesterol, in polyunsaturated membranes: microdomain formation. Biophys. J. 82:285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kariel, N., E. Davidson, and K. M. W. Keough. 1991. Cholesterol does not remove the gel-liquid crystalline phase transition of phosphatidylcholines containing two polyenoic acyl chains. Biochim. Biophys. Acta. 1062:70–76. [DOI] [PubMed] [Google Scholar]
- 18.Orädd, G., and G. Lindblom. 2004. Lateral diffusion studied by pulsed field gradient NMR on oriented lipid membranes. Magn. Reson. Chem. 42:123–131. [DOI] [PubMed] [Google Scholar]
- 19.Filippov, A., G. Orädd, and G. Lindblom. 2003. The effect of cholesterol on the lateral diffusion of phospholipids in oriented bilayers. Biophys. J. 84:3079–3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Filippov, A., G. Orädd, and G. Lindblom. 2004. Lipid lateral diffusion in ordered and disordered phases in raft mixtures. Biophys. J. 86:891–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filippov, A., G. Orädd, and G. Lindblom. 2006. Sphingomyelin structure influences the lateral diffusion and raft formation in lipid bilayers. Biophys. J. 90:2086–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindblom, G., G. Orädd, and A. Filippov. 2006. Lipid lateral diffusion in bilayers with phosphatidylcholine, sphingomyelin and cholesterol. An NMR study of dynamics and lateral phase separation. Chem. Phys. Lipids. 141:179–184. [DOI] [PubMed] [Google Scholar]
- 23.Orädd, G., P. W. Westerman, and G. Lindblom. 2005. Lateral diffusion coefficients of separate lipid species in a ternary raft-forming bilayer: a pfg-NMR multinuclear study. Biophys. J. 89:315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shahedi, V., G. Orädd, and G. Lindblom. 2006. Domain-formation in DOPC/SM bilayers studied by pfg-NMR: effect of sterol structure. Biophys. J. 91:2501–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanner, J. E. 1970. Use of the stimulated echo in NMR diffusion studies. J. Chem. Phys. 52:2523–2526. [Google Scholar]
- 26.Stilbs, P., K. Paulsen, and P. C. Griffiths. 1996. Global least-squares analysis of large, correlated spectral data sets: application to component-resolved FT-PGSE NMR spectroscopy. J. Phys. Chem. 100:8180–8189. [Google Scholar]
- 27.Lindblom, G., and G. Orädd. 2007. Order and disorder in a liquid crystalline bilayer: pulsed field gradient NMR studies of lateral phase separation. J. Dispersion Sci. Technol. 28:55–61. [Google Scholar]
- 28.Zhou, Z., B. G. Sayer, R. E. Stark, and R. M. Epand. 1997. High-resolution magic-angle spinning H-1 nuclear magnetic resonance studies of lipid dispersions using spherical glass ampoules. Chem. Phys. Lipids. 19:45–53. [Google Scholar]
- 29.Vaz, W. L. C., R. M. Clegg, and D. Hallmann. 1985. Translational diffusion of lipids in liquid crystalline phase phosphatidylcholine multibilayers. A comparison of experiment with theory. Biochemistry. 24:781–786. [DOI] [PubMed] [Google Scholar]
- 30.Almeida, P. F. F., W. L. C. Vaz, and T. E. Thompson. 1992. Lateral diffusion in the liquid phases of dimyristoilphosphatidylcholine/cholesterol bilayers: a free volume analysis. Biochemistry. 31:6739–6747. [DOI] [PubMed] [Google Scholar]
- 31.Cohen, M., and D. Turnbull. 1959. Molecular transport in liquids and gases. J. Chem. Phys. 31:1164–1169. [Google Scholar]
- 32.Clegg, R. M., and W. L. C. Vaz. 1985. Translational diffusion of proteins and lipids in artificial lipid bilayer membranes. A comparison of experiment with theory. In Progress in Protein-Lipid Interactions. Elsevier Science Publishers B.V., New York. 173–229.
- 33.Koenig, B. W., H. H. Strey, and K. Gawrisch. 1997. Membrane lateral compressibility determined by NMR and x-ray diffraction: effect of acyl chain polyunsaturation. Biophys. J. 73:1954–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemmich, J., K. Mortensen, J. H. Ipsen, T. Honger, R. Bauer, and O. G. Mouritsen. 1997. The effect of cholesterol in small amounts on lipid-bilayer softness in the region of the main phase transition. Eur. Biophys. J. 25:293–304. [DOI] [PubMed] [Google Scholar]
- 35.Trouard, T. P., A. A. Nevzorov, T. M. Alam, C. Job, J. Zajicek, and M. F. Brown. 1999. Influence of cholesterol on dynamics of dimyristoylphosphatidylcholine bilayers as studied by deuterium NMR relaxation. J. Chem. Phys. 110:8802–8818. [Google Scholar]
- 36.Wassall, S. R., M. R. Brzustowicz, S. R. Shaikh, V. Cherezov, M. Caffrey, and W. Stillwell. 2004. Order from disorder, corralling cholesterol with chaotic lipids. The role of polyunsaturated lipids in membrane raft formation. Chem. Phys. Lipids. 132:79–88. [DOI] [PubMed] [Google Scholar]
- 37.Eriksson, P. O., and G. Lindblom. 1993. Lipid and water diffusion in bicontinuous cubic phases measured by NMR. Biophys. J. 64:129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oradd, G., G. Lindblom, and P. W. Westerman. 2002. Lateral diffusion of cholesterol and dimyristoylphosphatidylcholine in a lipid bilayer measured by pulsed field gradient NMR spectroscopy. Biophys. J. 83:2702–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veatch, S. L., and S. L. Keller. 2005. Miscibility phase diagrams of giant vesicles containing sphingomyelin. Phys. Rev. Lett. 94:148101–148104. [DOI] [PubMed] [Google Scholar]
- 40.Veatch, S. L., I. V. Polozov, K. Gawrisch, and S. L. Keller. 2004. Liquid domains in vesicles investigated by NMR and fluorescence microscopy. Biophys. J. 86:2910–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Almeida, R. F. M., A. Fedorov, and M. Prieto. 2003. Sphingomyelin/phosphatidylcholine/cholesterol phase diagram: boundaries and composition of lipid rafts. Biophys. J. 85:2406–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Veatch, S. L., K. Gawrisch, and S. L. Keller. 2006. Closed-loop miscibility gap and quantitative tie-lines in ternary membranes containing diphytanoyl PC. Biophys. J. 90:4428–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]










