Abstract
In the next 15–30 years, manned space flight to Mars, our planetary neighbour, will become a reality and astronauts are likely to spend at least 2–3 years away from Earth. Time spent in such extreme environments will result in a diminution of immune status and profound changes in the human bacterial microflora. In microgravity, the efficacy of antibiotics is reduced and microbial mutation rates increase dramatically. These factors will impinge on the capacity to treat effectively the infections that will doubtless arise during such long and stressful endeavour. We highlight new rationales for the treatment of infectious disease that may be applicable to therapy in extreme environments such as deep space.
Keywords: Space travel, Bacterial infection, Intestinal flora, Mars missions, Photodynamic therapy, Bacteriophage therapy
1. Introduction
In early 2004, President George W. Bush committed the United States to a long-term human and robotic programme to explore the solar system. A new spacecraft, the Crew Exploration Vehicle, will be developed to undertake manned lunar missions by 2015, with the goal of living and working on the Moon for increasingly extended periods of time. The experience and knowledge gained from this venture will serve as a foundation for manned missions beyond Earth's orbit, beginning with Mars around the year 2020. It is thought extremely unlikely that missions will simply land astronauts on the planet and then return them to Earth, but will involve extensive exploration of the Martian surface [1]. Owing to the limitations of current propulsion designs, missions are likely to be launched either from the lunar surface or from the International Space Station, with outbound and inbound flight durations of ca. 6 months each and a projected surface stay of up to 570 days [2].
Such ventures into deep space will present an enormous challenge to those responsible for the provision of biomedical support. Missions beyond Earth's orbit entail unknown risks, there are no validated effective responses to many of the known risks that are likely to be encountered, and astronauts will be isolated without the capacity for real-time communication with Earth or a timely return [2]. Health risks associated with the numerous long-term near-Earth orbital flights that have been undertaken include microgravity-induced decreases in bone mineral density, psychological problems due to isolation, noise, confinement and a restricted working environment, and prolonged exposure to ionising and non-ionising forms of radiation [3]. It is inevitable that these risks will be exacerbated when astronauts travel on longer missions beyond Earth's orbit. In particular, flight beyond the Earth's magnetosphere exposes both men and spacecraft equipment to a continuous flux of galactic cosmic rays, consisting of protons and high-energy heavy nuclei, and occasional but intense fluxes of solar energetic particles, primarily protons and alpha particles.
2. Infection in space travel
Analysis of medical events among astronauts aboard the Russian space station Mir over a period from March 1995 to June 1998 indicated that a significant number of episodes of microbial infection occurred, including conjunctivitis and acute respiratory and dental infections [2]. In addition, injury and trauma, such as lacerations and open fractures, are likely to occur on long missions and will require prophylactic administration of antibiotics to prevent serious wound infection. Assuming no significant microbial contamination of the fabric of the spacecraft or its air, food and water supply, any infections in crew members will result from endogenous human and animal flora that are brought on board at the time of departure and this is likely to limit the types of infection encountered in a way that should be predictable. Changes in the composition of the intestinal [4], oral [5,6] and nasal [6] bacterial microflora have been noted after short space flights and may be diet-related, although some evidence has been found for in-flight cross-contamination with opportunistic pathogens such as Staphylococcus aureus in the upper respiratory tract [6]. This latter observation supports the conclusion of an analysis of the effect of space flight on the nasal flora of Soviet cosmonauts that there is sometimes a reduction in the number of non-pathogenic bacteria and an increase in the number of opportunistic pathogens [7]. Opportunities for bacteria to establish foci of infection will be enhanced due to the negative impact of space travel on immune function, in particular cell-mediated immunity [8].
By far the largest microbial reservoir on board will be the resident intestinal flora of the crew. As early as 2 weeks into the confinement imposed by space flight there is a significant reduction in the number of bacterial species that can be isolated from the intestinal tract as well as an interchange of intestinal bacteria between crew [8]. These observations parallel terrestrial experiments in which volunteers were kept in isolation and fed only sterilised, dehydrated foods: such conditions led to sharp decreases in the numbers of bifidobacteria, lactobacilli and other bacteria considered beneficial to health. The ingestion of bacteria present in food appears to assist in the maintenance of a healthy and diverse intestinal flora, and removal of this exogenous microbial source is likely to lead to undesirable changes that will affect immunocompetence and nutritional status, increasing the risk of opportunistic infections, poor wound healing and metastatic disease. Such large shifts in the composition of the commensal flora may also provide opportunities for the dissemination and spread of antibiotic resistance genes. These effects might be circumvented, or at least ameliorated, by regular intake of probiotic foods.
3. Emergence of resistance
Modifications to the bacterial phenotype leading to reversible increases in antibiotic resistance are known to occur during short-term space flight. Staphylococcus aureus and Escherichia coli isolates obtained from the commensal flora of the French astronaut J.L. Chrétien were studied aboard Salyut 7 in July 1982 to determine the effects of space flight on bacterial ultrastructure and antibiotic sensitivity [9,10]. Minimum inhibitory concentrations (MICs) of the E. coli isolate against both colistin and kanamycin increased significantly (from 4 mg/L to >16 mg/L) when the bacteria were grown and tested aboard the flight module compared with control experiments conducted on the ground. There were smaller increases in MICs of the S. aureus isolate against oxacillin, erythromycin and chloramphenicol, although very large increases in the thickness of the staphylococcal cell wall were noted following in-flight growth in the absence of antibiotic. Interestingly, these profound changes in cell wall morphology are very similar to those observed in vancomycin intermediate-resistant S. aureus [11,12] and in polyphenol-grown methicillin-resistant S. aureus [13]. In both these cases, cell wall thickening is intimately associated with changes in sensitivity to antibiotics. The phenotypic nature of flight-induced changes was established by terrestrial culture; antibiotic sensitivity and gross morphology reverted to pre-flight values when in-flight cultures were subcultured in a terrestrial environment. These changes were confirmed and extended through further experiments conducted aboard the American shuttles Challenger [14] and Discovery [15] in November 1985 and January 1992, respectively. The Challenger experiments established that in-flight-cultivated E. coli ATCC 25992 grew more rapidly in subinhibitory concentrations of colistin than cells cultured in a terrestrial environment. The same strain grew more rapidly in the presence of subinhibitory concentrations of dihydrostreptomycin in-flight compared with terrestrial culture. Thus, it appears that the efficacy of antibiotics may be compromised during even short orbital missions.
Reduction in the diversity of the bacterial flora of the gastrointestinal tract may give rise to an increase in the size of the drug resistance gene pool if those biotypes that come to predominate carry antibiotic resistance determinants. Emergence of such resistant clones may be enhanced by selective antibiotic pressure either before or during the flight and it will be important to screen the microflora of astronauts prior to launch to reduce the potential for emergence of resistant biotypes. Emergence of resistance may also be facilitated by mutation and it has been noted that long-term space flight engendered high mutation frequencies in indicator genes carried by yeast: after a 40 day flight aboard Mir, mutation rates for a cloned bacterial ribosomal gene were two to three times higher than those for ground samples [16]. Although mutation frequencies were measured in artificial genetic constructs, were higher than would be anticipated in wild-type genes and were deletion mutations rather than point mutations, this study implies that space radiation containing high linear-energy transfer may provide further scope for the emergence of drug resistance in the bacterial microflora. Against such a background, even the prudent use of conventional antibiotics during extended space flight may result in the rapid emergence of resistant genotypes and compromise effective chemotherapy with what must of necessity be a restricted on-board pharmacy. It is likely that resistant forms would rapidly colonise all members of the crew and come to predominate in the microflora, acting as a potential reservoir for difficult-to-treat opportunistic infections. New therapeutic modalities that suppress or abrogate the emergence of antibiotic-resistant forms in such a setting have obvious attractions.
4. Photosensitisation
Dosing of high concentrations of two mechanistically unrelated antibiotics has been advocated as a procedure for slowing the rate of emergence of antibiotic resistance from susceptible populations [17], although conventional drugs used in this way may have further adverse effects on the composition of the microflora unless both agents have a narrow spectrum of activity. With conventional antibacterial agents, resistance follows use, and we need to find ways of breaking this cycle. Most antibiotics kill or inhibit bacteria by interfering with or disrupting biosynthetic pathways. These discrete modes of action appear to present the bacterial cell with an opportunity to bypass the susceptible metabolic step, to prevent the antibiotic reaching the target, or to produce an enzyme that breaks down the antibiotic before it can inhibit cellular processes, and many such mechanisms have evolved through the heavy selective pressure of antibiotic use. Therapeutic modalities producing generalised damage to bacteria, rather than discretely inhibiting one or two biochemical targets or metabolic steps, should discourage the emergence of resistant genotypes. In recent years there has been renewed interest in the application of photosensitive agents to the treatment of topical infections [18]. Tetrapyrrole dyes such as porphyrins, phthalocyanines and bacteriochlorins are able to accumulate in and be selectively retained by abnormal cells and by bacteria. Activation of these molecules with visible light in the presence of oxygen leads to the destruction of the target tissue by singlet oxygen-mediated peroxidative damage. Singlet oxygen is very toxic to cells but is very short-lived and causes only local damage. A variety of macromolecular structures within the bacterial cell are rendered non-functional by this process. Pre-clinical studies indicate that this approach effectively eliminates bacteria from sites of superficial infection, such as wound [19] and oral [20] infections, without significant side effects. This broad-ranging therapeutic approach is effective against multidrug-resistant bacteria [18] and biofilms [21] and could provide an effective means of treating superficial but troublesome infections on extended flights with little impact on the size of the on-board pharmacy. Indeed, the use of on-board light sources may have more general benefits and offset the biological effects of prolonged exposure to microgravity, which include slow wound healing, changes in whole-body blood distribution and suppression of the immune response. These ‘microgravity effects’ could be partly counteracted through the use of lasers or arrays of light-emitting diodes of appropriate wavelength and power that are known to engender biostimulatory effects at both the cellular and systemic level [22,23].
5. Phage therapy
The use of bacteriophages as therapeutic agents during extensive periods of isolation beyond the reach of terrestrial agencies could also be considered. These bacterial viruses propagate within and lyse their bacterial host and are gaining attention as viable alternatives to conventional chemotherapy [24]. Bacteriophage therapy has several potential advantages: each bacteriophage attacks only a very limited range of bacteria, and they are almost always specific for a bacterial species. They can therefore be targeted to specific disease-causing bacteria with little disturbance to the normal bacterial flora of the host. They are easily grown and purified. Small doses can be given because they are ‘living medicines’ and their numbers increase as they spread among the target bacterial population. They do not appear to be toxic—they invade only bacteria and not human cells. They are self-limiting—once the target population is destroyed, phage numbers reduce rapidly. As they are an assemblage of foreign proteins and nucleic acid, they evoke a substantial immune response when administered into the blood circulation and are considered primarily as therapeutics for non-systemic infections, including wounds, abscesses, and gastrointestinal, skin and pulmonary infections [25]. Resistance to the bacteriophage can emerge, sometimes during therapy, but bacteriophage variants that attack the new form may be readily isolated using relatively simple procedures that could be adapted for space flight.
6. New strategies
Conventional antibiotics facilitate the emergence and dissemination of antibiotic resistance genes and thus alter the genetic makeup of bacterial populations. This powerful selective pressure could be reduced or even eliminated by the use of agents that do not kill pathogenic bacteria but modify them to produce a ‘less fit’ phenotype unable to survive at the site of infection [26,27]. Bacteria survive, multiply and cause infection because they are either virulent (producing factors that enable them to survive and cause the symptoms of disease) or resistant to antibiotics (producing factors that enable them to survive the onslaught of antibiotic therapy). Agents have been identified that can sensitise bacteria to a previously ineffective antibiotic or to a component of the host's immune system. Such modifying agents may slow down or prevent the emergence of resistant genotypes and could form the basis of an effective strategy for the management of a broad range of on-board infections. Two examples will serve to illustrate the potential of this approach.
Many pathogenic bacteria produce hydrated, negatively charged polysaccharide capsules external to the cell wall; these structures confer resistance to immune mechanisms such as engulfment by phagocytes and killing by complement [28]. Encapsulated bacteria cause severe infections such as septicaemia, meningitis, pneumonia, osteomyelitis, septic arthritis and pyelonephritis, as well as less severe non-invasive infections. Often, the capsule is the major determinant of survival and confers on the pathogen the capacity to cause infection. Removing the capsule, or inhibiting its biosynthesis, may represent an alternative to conventional chemotherapy, by sensitising the bacteria to components of the host's defences. Bacteriophages that attack encapsulated bacteria frequently carry an enzyme that enables them to work their way through the protective polysaccharide layer and make contact with the surface proper [29]. Could such enzymes be used in vivo to strip away the capsule and expose the underlying bacterial surface to immune attack? We have used capsule depolymerases as therapeutics in animals infected with encapsulated pathogens and found that they have a profound effect on the course of potentially fatal infection. For example, intraperitoneal administration of small quantities of capsule-specific enzyme prevented and cured systemic infection due to polysialic acid (PSA)-coated E. coli in young rats [30,31]. Once the protective PSA layer is removed from the outer surface, the attenuated bacteria become exquisitely susceptible both to complement-mediated killing [30] and to macrophage uptake [31].
Can we modify drug-resistant bacteria to prevent the expression of resistance determinants? Such an approach could revive the use of older antibiotics that have become less effective due to the emergence and spread of resistance and thus protect the efficacy of the on-board pharmacy. There is little doubt that compounds that restore the sensitivity of major pathogens to established antibiotics would be invaluable therapeutic aids. With this in mind, we investigated the capacity of components of green tea to modulate the expression of proteins critical for the determination of β-lactam resistance in S. aureus [27,32]. Resistance to β-lactam drugs such as methicillin and oxacillin (developed in response to the spread of resistance to earlier penicillins) is now a common trait in this bacterium. Methicillin resistance arises due to the expression by the bacterium of a modified penicillin binding protein (PBP2a) to which β-lactams bind only poorly. Extracts of green tea have been shown to restore the sensitivity of methicillin-resistant S. aureus (MRSA) to methicillin and similar antibiotics [33]. Some components of green tea, particularly epicatechin gallate (ECg), are able to sensitise MRSA to β-lactam antibiotics through interference with the activity of PBP2a in the staphylococcal membrane [34]. Thus, a β-lactam such as oxacillin together with a pharmacologically acceptable derivative of ECg has the potential to provide a therapeutic combination for the treatment of MRSA infection (Fig. 1). In addition, ECg has the capacity to inhibit the secretion of toxins and other virulence factors elaborated by staphylococci, therefore such modulators could have wide application not least because if administered as the primary therapeutic agent, they would not directly select for the drug-resistant genotype.
Fig. 1.
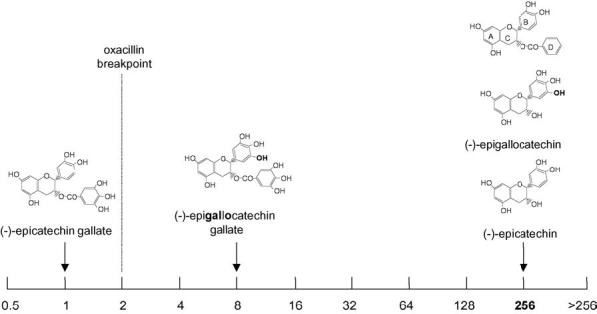
Oxacillin minimum inhibitory concentration (MIC) (μg/mL) for oxacillin used in combination with catechin derivatives (25 μg/mL) for constitutive penicillin-binding protein 2a (PBP2a) producer S. aureus BB568. Oxacillin MIC in the absence of catechin was 256 μg/mL. Courtesy of Dr Paul Stapleton, London School of Pharmacy.
7. Conclusions
Astronauts selected for future missions to Mars will have a very high level of intrinsic fitness but they will be subjected to a range of stresses that over time will compromise immune function [35]. A crew will carry with them a sizeable pool of microbial genotypes from which may emerge antibiotic-resistant bacteria that could cause serious, difficult-to-treat infections. Such a scenario could threaten the future of the mission. Antibiotics included in the on-board pharmacy could become redundant if drug-resistant populations of opportunistic bacterial pathogens become dominant within their microflora. The range of drugs carried will of necessity be limited, but the provision of agents such as those described herein that suppress the emergence of antibiotic-resistant forms could provide a formidable armamentarium against the constant threat of infection.
Acknowledgments
P.W.T. thanks the Medical Research Council and the British Society for Antimicrobial Chemotherapy for financial support.
References
- 1.Hoffman SJ, Kaplan DI. Human exploration of Mars: the reference mission of the NASA Mars exploration study team. Houston, TX: National Aeronautics and Space Administration; 1997. (NASA Special Publications 6107). [Google Scholar]
- 2.Ball JR, Evans CH. Safe passage: astronaut care for exploration missions. Washington, DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- 3.Grigoriev AI, Kozlovskaya IB, Potapov AN. Goals of biomedical support of a mission to Mars and possible approaches to achieving them. Aviat Space Environ Med. 2002;73:379–84. [PubMed] [Google Scholar]
- 4.Lencner AA, Lencner CP, Mikelsaar ME, et al. Die quantitative Zusammensetzung der Lactoflora des Verdauungstrakt vor und nach kosmischen Flügen unterschiedlicher Dauer. Nahrung. 1984;28:607–13. doi: 10.1002/food.19840280608. [DOI] [PubMed] [Google Scholar]
- 5.Brown LR, Fromme WJ, Handler SF, Wheatcroft MG, Johnston DA. Effect of Skylab missions on clinical and microbiologic aspects of oral health. J Am Dent Assoc. 1976;93:357–63. doi: 10.14219/jada.archive.1976.0502. [DOI] [PubMed] [Google Scholar]
- 6.Decelle JG, Taylor GR. Autoflora in the upper respiratory tract of Apollo astronauts. Appl Environ Microbiol. 1976;32:659–65. doi: 10.1128/aem.32.5.659-665.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nefedov YG, Shilov VM, Koustantinova IV, Zaloguev SN. Microbiological and immunological aspects of extended manned space flights. Life Sci Space Res. 1971;9:11–6. [PubMed] [Google Scholar]
- 8.Hales NW, Yamauchi K, Alicea A, Sundaresan A, Pellis NR, Kulkarni AD. A countermeasure to ameliorate immune dysfunction in in vitro simulated microgravity environment: role of cellular nucleotide nutrition. In Vitro Cell Dev Biol Anim. 2002;38:213–7. doi: 10.1290/1071-2690(2002)038<0213:ACTAID>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 9.Tixador R, Richoilley G, Gasset G, et al. Preliminary results of Cytos 2 experiment. Acta Astronaut. 1985;12:131–4. doi: 10.1016/0094-5765(85)90082-7. [DOI] [PubMed] [Google Scholar]
- 10.Tixador R, Richoilley G, Gasset G, et al. Study of minimal inhibitory concentration of antibiotics on bacteria cultured in vitro in space (Cytos 2 experiment) Aviat Space Environ Med. 1985;56:748–51. [PubMed] [Google Scholar]
- 11.Sieradzki K, Pinho MG, Tomasz A. Inactivated pbp4 in highly glycopeptide-resistant laboratory mutants of Staphylococcus aureus. J Biol Chem. 1999;274:18942–6. doi: 10.1074/jbc.274.27.18942. [DOI] [PubMed] [Google Scholar]
- 12.Sieradzki K, Tomasz A. Alterations of cell wall structure and metabolism accompany reduced susceptibility to vancomycin in an isogenic series of clinical isolates of Staphylococcus aureus. J Bacteriol. 2003;185:7103–10. doi: 10.1128/JB.185.24.7103-7110.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamilton-Miller JMT, Shah S. Disorganisation of cell division of methicillin-resistant Staphylococcus aureus by a component of tea (Camellia sinensis): a study by electron microscopy. FEMS Microbiol Lett. 1999;176:463–9. doi: 10.1111/j.1574-6968.1999.tb13698.x. [DOI] [PubMed] [Google Scholar]
- 14.Lapchine L, Moatti N, Gasset G, Richoilley G, Templier J, Tixador R. Antibiotic activity in space. Drugs Exp Clin Res. 1985;12:933–8. [PubMed] [Google Scholar]
- 15.Tixador R, Gasset G, Moatti N, et al. Behavior of bacteria and antibiotics under space conditions. Aviat Space Environ Med. 1994;65:551–6. [PubMed] [Google Scholar]
- 16.Fukada T, Fukada K, Takahashi A, et al. Analysis of deletion mutations of the rpsL gene in the yeast Saccharomyces cerevisiae detected after long-term flight on the Russian space station Mir. Mutat Res. 2000;470:125–32. doi: 10.1016/s1383-5742(00)00054-5. [DOI] [PubMed] [Google Scholar]
- 17.Drlica K. A strategy for fighting antibiotic resistance. ASM News. 2001;67:27–33. [Google Scholar]
- 18.Wainwright M. Photodynamic antimicrobial chemotherapy (PACT) J Antimicrob Chemother. 1998;42:13–28. doi: 10.1093/jac/42.1.13. [DOI] [PubMed] [Google Scholar]
- 19.Hamblin MR, O'Donnell DA, Murthy N, Contag CH, Hasan T. Rapid control of wound infections by targeted photodynamic therapy monitored by in vivo bioluminescence imaging. Photochem Photobiol. 2002;75:51–7. doi: 10.1562/0031-8655(2002)075<0051:rcowib>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 20.Wilson M. Lethal photosensitisation of oral bacteria and its potential application in the photodynamic therapy of oral infections. Photochem Photobiol Sci. 2004;3:412–8. doi: 10.1039/b211266c. [DOI] [PubMed] [Google Scholar]
- 21.Wilson M, Burns T, Pratten JJ. Killing of Streptococcus sanguis in biofilms using a light-activated antimicrobial agent. J Antimicrob Chemother. 1996;37:377–81. doi: 10.1093/jac/37.2.377. [DOI] [PubMed] [Google Scholar]
- 22.Sommer AP, Pinheiro ALB, Mester AR, Franke RP, Whelan HT. Biostimulatory windows in low-intensity laser activation: lasers, scanners, and NASA's light-emitting diode array system. J Clin Laser Med Surg. 2001;19:29–33. doi: 10.1089/104454701750066910. [DOI] [PubMed] [Google Scholar]
- 23.Sommer AP, Oron U, Kajander EO, Mester AR. Stressed cells survive better with light. J Proteome Res. 2002;1:475. doi: 10.1021/pr0255396. [DOI] [PubMed] [Google Scholar]
- 24.Chanishvili N, Chanishvili T, Tediashvili M, Barrow PA. Phages and their application against drug-resistant bacteria. J Chem Technol Biotechnol. 2001;76:689–99. [Google Scholar]
- 25.Merril CR, Scholl D, Adhya SL. The prospect for bacteriophage therapy in Western medicine. Nat Rev Drug Discov. 2003;2:489–97. doi: 10.1038/nrd1111. [DOI] [PubMed] [Google Scholar]
- 26.Tan Y-T, Tillett DJ, McKay IA. Molecular strategies for overcoming antibiotic resistance in bacteria. Mol Med Today. 2000;6:309–14. doi: 10.1016/s1357-4310(00)01739-1. [DOI] [PubMed] [Google Scholar]
- 27.Taylor PW, Stapleton PD, Luzio JP. New ways to treat bacterial infections. Drug Discov Today. 2002;7:1086–91. doi: 10.1016/s1359-6446(02)02498-4. [DOI] [PubMed] [Google Scholar]
- 28.Cross AS. The biologic significance of bacterial encapsulation. Curr Top Microbiol Immunol. 1990;150:87–95. doi: 10.1007/978-3-642-74694-9_5. [DOI] [PubMed] [Google Scholar]
- 29.Hughes KA, Sutherland IW, Clark J, Jones MV. Bacteriophage and associated polysaccharide depolymerases—novel tools for study of bacterial biofilms. J Appl Microbiol. 1998;85:583–90. doi: 10.1046/j.1365-2672.1998.853541.x. [DOI] [PubMed] [Google Scholar]
- 30.Mushtaq N, Redpath MB, Luzio JP, Taylor PW. Prevention and cure of systemic Escherichia coli K1 infection by modification of the bacterial phenotype. Antimicrob Agents Chemother. 2004;48:1503–8. doi: 10.1128/AAC.48.5.1503-1508.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mushtaq N, Redpath MB, Luzio JP, Taylor PW. Treatment of experimental Escherichia coli infection with recombinant bacteriophage-derived capsule depolymerase. J Antimicrob Chemother. doi: 10.1093/jac/dki177. in press. [DOI] [PubMed] [Google Scholar]
- 32.Stapleton PD, Taylor PW. Methicillin resistance in Staphylococcus aureus: mechanisms and modulation. Sci Prog. 2002;85:57–72. doi: 10.3184/003685002783238870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamilton-Miller JMT, Shah S. Activity of the tea component epicatechin gallate and analogues against methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 2000;46:847–63. doi: 10.1093/jac/46.5.852. [DOI] [PubMed] [Google Scholar]
- 34.Stapleton PD, Shah S, Anderson JC, Hara Y, Hamilton-Miller JMT, Taylor PW. Modulation of β-lactam resistance in Staphylococcus aureus by catechins and gallates. Int J Antimicrob Agents. 2004;23:462–7. doi: 10.1016/j.ijantimicag.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 35.Sommer AP, Wickramasinghe NC. Keeping nanobacterial infections at bay during space travel. Int J Antimicrob Agents. 2004;24:548–9. doi: 10.1016/j.ijantimicag.2004.09.006. [DOI] [PubMed] [Google Scholar]


