Abstract
Owing to multiple anatomical connections and functional interactions between the habenulo-interpeduncular and the mesolimbic pathways, it has been proposed that these systems could together mediate the reinforcing properties of addictive drugs. 18-Methoxycoronaridine, an agent that reduces morphine self-administration and attenuates dopamine sensitization in the nucleus accumbens in response to repeated morphine, has been shown to produce these effects by acting in the medial habenula and interpeduncular nucleus. Acetylcholine, one of the predominant neurotransmitters in the interpeduncular nucleus, may be a major determinant of these interactions. To determine if and how morphine acts in the interpeduncular nucleus, the effects of acute and repeated administration of morphine on extracellular acetylcholine levels in this brain area were assessed. In addition, the motor behavior of rats receiving repeated morphine administration was monitored during microdialysis sessions. Acutely, morphine produced a biphasic effect on extracellular acetylcholine levels in the interpeduncular nucleus such that low and high doses of morphine (i.e., 5 and 20 mg/kg i.p.) significantly increased and decreased acetylcholine levels, respectively. Repeated administration of the same doses of morphine resulted in tolerance to the inhibitory but not to the stimulatory effects; tolerance was accompanied by sensitization to morphine-induced changes in locomotor activity and stereotypic behavior. The latter results suggest that tolerance to morphine's effect on the cholinergic habenulo-interpeduncular pathway is related to its sensitizing effects on the mesostriatal dopaminergic pathways.
Keywords: opioids, habenulo-interpeduncular pathway, microdialysis, cholinergic, liquid chromatography, habenula
1. Introduction
The habenulo-interpeduncular pathway, a part of the dorsal diencephalic conduction system (Sutherland, 1982), conveys primarily cholinergic fibers in the core of the fasciculus retroflexus from the medial habenula in the diencephalon to the interpeduncular nucleus (IPN) in the midbrain (Morley, 1986). The habenulointerpeduncular pathway was described by Blander and Wise (1989) as a distinct system supporting self-stimulation reward, separate from the better known mesolimbic pathway in the medial forebrain bundle. Several studies in the 1980's focused on the interactions of the mesolimbic and the habenulo-interpeduncular pathways and provided evidence of their mutual inhibitory relationship (Sutherland and Nakajima, 1981;Nishikawa et al., 1986). Thus, rates of habenular self-stimulation were increased in rats with ipsilateral lesions of the medial forebrain bundle (Sutherland and Nakajima, 1981). Furthermore, dopamine metabolism in the nucleus accumbens was increased after infusion of tetrodotoxin into the fasciculus retroflexus (Nishikawa et al., 1986). Taken collectively, these studies suggested that the habenulo-interpeduncular and the mesolimbic pathways jointly mediate the rewarding effects of natural reinforcers and of addictive drugs (Ellison, 1994). Consistent with this premise, recent findings from our laboratory have suggested that the self-administration of morphine as well as morphine-induced sensitization of the mesolimbic dopaminergic pathway can be modulated by nicotinic antagonists acting in the habenulo-interpeduncular pathway (Glick et al., 2006;Taraschenko et al., 2005).
Sensitization of the mesolimbic dopaminergic pathway in response to drugs of abuse is believed to be a neurochemical substrate for persistent drug-seeking behavior and relapse in human addicts (Robinson and Berridge, 1993). During repeated administration of morphine, sensitized dopamine responses are mediated by several adaptations in glutamate and dopamine neurotransmission, both in the nucleus accumbens and in the ventral tegmental area (Vanderschuren and Kalivas, 2000). Altered functioning of other brain areas, including the habenulo-interpeduncular pathway, may also occur.
Several studies on effects of opioids on the habenulo-interpeduncular pathway have focused on opioid-induced changes in c-Fos immunoreactivity and glucose utilization (Bot and Chahl, 1996;Martin et al., 1997). However, the effect of opioids on cholinergic neurotransmission in the habenulo-interpeduncular pathway have not previously been assessed. The present study was designed to determine how morphine affects the neurochemistry of the habenulo-interpeduncular pathway and if such effects are related to motor behavior presumably mediated by the mesostriatal pathways. Thus, the release of acetylcholine in the interpeduncular nucleus in rats was measured in response to acute and repeated administration of morphine while simultaneously assessing motor behavior.
2. Materials and Methods
2.1 Animals
Experiments were conducted in accordance with the “Guide for the Care and Use of Laboratory Animals” (1996) and were approved by the Institutional Animal Care and Use Committee of Albany Medical College. Naïve female Sprague-Dawley rats (Taconic, Germantown, NY), weighing 250-310 g, were housed individually and maintained on a normal 12:12-h light/dark cycle (light on 7 a.m., light off at 7 p.m.). Food and water were provided ad libitum.
2.2 Drugs
Morphine sulfate (Research Biochemicals Inc., Natick, MA) was dissolved in saline and injected intraperitoneally. Drug solutions were made fresh for each experiment.
2.3 Stereotaxic brain cannulation surgery
The surgery was performed according to the previously described protocol (Maisonneuve and Glick, 1999). Coordinates in mm were chosen according to Paxinos and Watson (1986) such that when a dialysis probe(s) were inserted, the tip was located in the interpeduncular nucleus (in mm, AP=−6.3; ML=±2.6; DV=−9.2 using a 15° angle). For the acute studies, the rats were implanted with one microdialysis guide cannula while for the repeated administration studies, the animals were implanted with bilateral guide cannulae above the interpeduncular nucleus. Rats were allowed to recover for three to five days before experiments were begun.
2.4 Microdialysis
2.4.1 Acute morphine studies
On the afternoon before the dialysis day, a calibrated 1-mm dialysis probe (CMA, North Chelmsford, MA) was inserted into the interpeduncular nucleus and continuously perfused (1 μl/min) with artificial cerebrospinal fluid (146 mM NaCl, 2.7 mM KCL, 1.2 mM CaCl2, 1.0 mM MgCl2, pH=7.3) containing 0.1μM neostigmine. The collection of brain perfusates began the next morning. Six twenty-minute baseline fractions and nine fractions following morphine (5, 10, 20 mg/kg i.p.) or saline administration were obtained and analyzed for acetylcholine with high performance liquid chromatography (HPLC). Upon completion of a microdialysis experiment, the animals were euthanized and their brains were processed for verification of probe placements.
2.4.2 Repeated morphine administration studies
The experiment was conducted using a previously described “within-subject” paradigm such that each rat was dialyzed twice, seven days apart, from opposite sides of the brain (Szumlinski et al., 2000). Specifically, on the day of the first microdialysis session, after collection of six baseline samples, rats received an injection of morphine (5 or 20 mg/kg i.p.); the effect of morphine was monitored for 3 h thereafter. Upon completion of a microdialysis session, the probe was removed from the brain, the rats were disconnected from the chamber and returned to their home cage. The cages were maintained in the colony room. Twenty four hours after the first injection of morphine, rats were brought back to the microdialysis room for an injection of morphine (5 or 20 mg/kg i.p.); rats were maintained in the same environment for 3 h afterwards and then subsequently returned to the colony room. This protocol was repeated daily for three subsequent days. This repeated morphine treatment procedure was previously utilized in our laboratory (Szumlinskiet al., 2000) and has been shown to promote the development of morphine-context associations in rats (Stewart et al., 1984). After 48 h of withdrawal, animals were prepared for the second microdialysis session and probes were inserted into the contralateral interpeduncular nucleus. On the following day, after collection of baseline samples, each rat received an injection of morphine (5 or 20 mg/kg i.p.). The effect of morphine was monitored for the following 3h. The samples from each dialysis session were analyzed immediately for acetylcholine with HPLC.
2.5 HPLC
The HLPC system consisted of an ESA 540 autosampler, an ESA solvent delivery system and an ESA Coulochem II electrochemical detector. Chromatograms were analyzed with an EZChrom Elite software (ESA). The mobile phase containing 50mM NaH2PO4, 0.5 mM Na2EDTA and 50 μl of 0.005% ProClin, adjusted to pH=8.5 with 6N NaOH, was pumped at a flow rate 120 μl/min. After separation in the analytical column (UniJet 50x1 mm, BAS, West Lafayette, IN), acetylcholine was enzymatically converted to hydrogen peroxide in the miniature post-column enzyme reactor (BAS) and electrochemically detected by an ESA 5041 analytical cell containing peroxidase-redox polymer-coated glassy carbon target electrode maintained at −200mV.
2.6 Verification of the probe sites
Upon completion of the microdialysis session, rats were euthanized in a CO2 chamber. Brains were rapidly removed and frozen at -80°C until thin-sectioned (40 μm). Only the data from animals with probes located within the boundaries of the interpeduncular nucleus were accepted for analysis (Fig.1).
Figure 1.
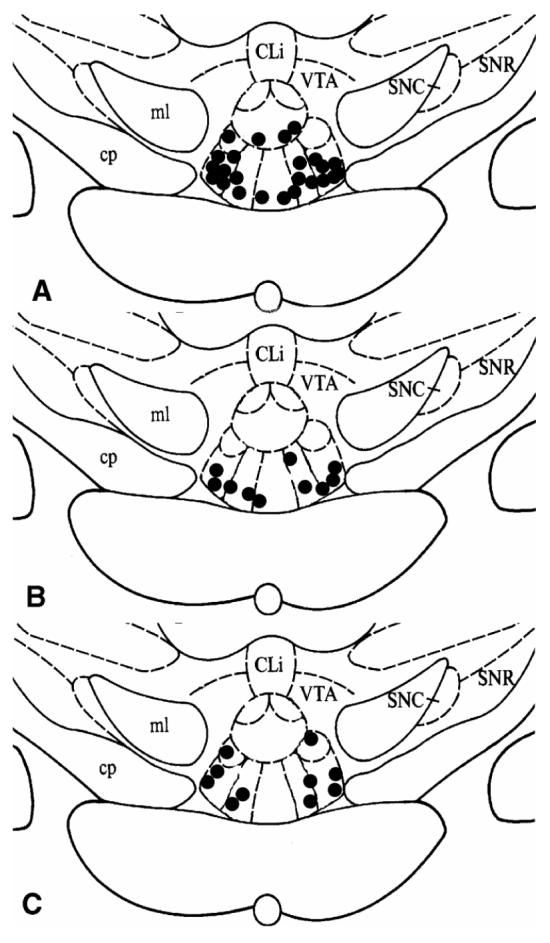
Localization of the tips of microdialysis probes in the interpeduncular nucleus of animals treated (n=24) with saline or acute morphine (A); animals treated (n=5) with repeated morphine, 5 mg/kg (B); and animals treated (n=5) with repeated morphine, 20 mg/kg (C). Cli, caudal linear raphe nucleus; SNC, substantia nigra compacta; SNR, substantia nigra reticular; VTA, ventral tegmental area; ml, medial lemniscus; cp, cerebral peduncle.
2.7 Behavioral studies
In repeated morphine administration experiments, the microdialysis sessions were videotaped and the behavior was later scored for the middle 6 min 40 sec of every 20-min interval corresponding to a dialysate sample. The behaviors assessed included gnawing, grooming and sniffing. Gnawing was defined as biting of the mesh floor of the microdialysis chamber or biting the hind paws. Grooming was defined as licking or cleaning any part of the body. Each behavior was scored as one incident for each 20-second interval. In addition, turning, rearing, stretching and locomotor activity were assessed and scored as one point per occurrence regardless of its duration. Turning was defined as rotating 360 °. Locomotor activity was defined as quadrant crossing by two-thirds of the body. Animals crossing a quadrant but returning backward to the previous quadrant received one point. If none of the above behaviors occurred during a 20-sec interval, one point of “immobile” behavior was assigned.
2.8 Statistical Analysis
For microdialysis experiments with acute morphine administration, the basal levels of extracellular acetylcholine were expressed as fmol/15 μl and were analyzed with repeated measures analysis of variance (ANOVA) with dose of morphine (0, 5, 10 and 20 mg/kg) as the main factor and time as a repeated measures variable. The morphine data were expressed as percent of appropriate basal means and analyzed with ANOVA as above. For the repeated morphine administration studies, the basal levels for both dialysis sessions were expressed as fmol/15 μl were and compared across weeks with repeated measures ANOVA using week as the main factor and time as repeated measure variable. In addition, the effects of repeated morphine were assessed across weeks as above with the morphine data expressed as percent of appropriate basal means. Post-hoc comparison tests (Fisher Least Squares Difference, LSD) were performed when appropriate.
For behavioral studies, the effects of acute morphine were assessed with repeated measures ANOVA with treatment as the main factor and time as a repeated measure variable. Furthermore, sensitization to chronic morphine administration was assessed with repeated measures ANOVA with the week as the main factor and the time as repeated measure variable.
3. Results
3.1 Acetylcholine release
3.1.1 Acute morphine studies
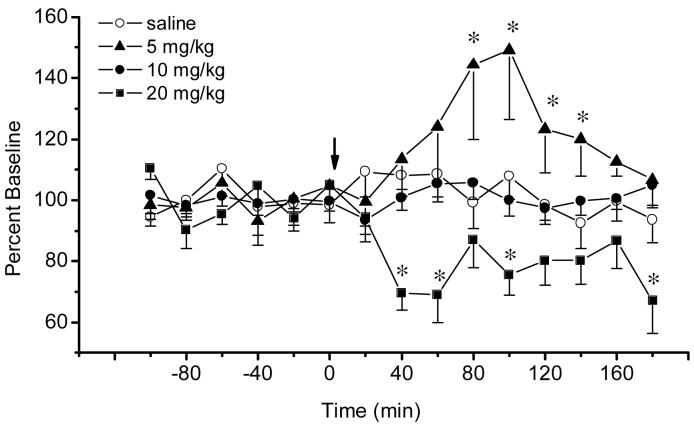
The microdialysis data accepted for analysis came from probes located from 6.04 mm to 6.72 mm posterior to bregma (Paxinos and Watson, 1986) and were positioned within the boundaries of the IPN (Fig.1A). The average basal level of extracellular acetylcholine in the IPN was 445.0 ± 65.6 fmols/15μl (mean ± SEM, n=24). There were no differences in basal concentrations among treatment groups (Group effect: F3, 20 =3.7 4, P>0.54). Vehicle injections did not significantly affect levels of acetylcholine in the interpeduncular nucleus (Time effect: F14, 70 = 0.84, P>0.60; Fig.2). The effects of three dosages of morphine (i.e. 5, 10 and 20 mg/kg i.p.) were assessed. As shown in Fig.2, morphine produced a biphasic effect on acetylcholine release, such that the lowest dose enhanced while the highest dose decreased acetylcholine levels (Group effect: F3, 20 = 6.74, P<0.003; Group x Time interaction F 42, 280 = 2.50, P<0.00001). Specifically, 5 mg/kg increased the release of acetylcholine at all time points from 80 to140 min after treatment with a peak increase of 149.1 % of baseline at 100 minutes. In contrast, 20 mg/kg decreased acetylcholine release at 40, 60, 100 and 180 min after injection; this decrease was maximal (69.1 % of baseline) at 60 minutes. The dose of 10 mg/kg did not alter the levels of acetylcholine in the interpeduncular nucleus.
Figure 2.
Time-course of extracellular acetylcholine (mean ± S.E.M.) in the interpeduncular nucleus in rats treated with acute morphine (5, 10 or 20 mg/kg, i.p., n=6) or saline (n=6). Arrow indicates injection of morphine or saline. *, P<0.05 morphine vs saline, Fisher LSD tests.
3.1.2 Repeated morphine administration studies
The histological data for animals having acceptable probe placements are shown in Fig.1B-C. The mean basal extracellular levels of acetylcholine were 517.497 ± 224.126 fmols/15μl and 378.059 ± 136.071 for week 1 and week 2 dialysis sessions, respectively (mean ± SEM, n=10). In the study with the low dose of chronic morphine (i.e. 5 mg/kg i.p.), the basal levels of extracellular acetylcholine in the IPN were not significantly altered as a result of repeated morphine administration (Week effect: F1, 4 = 0.28, P>0.62; Week × Time interaction F 5, 20 = 0.28, P>0.92). Likewise, in the study with the high dose of morphine (i.e., 20 mg/kg i.p.), basal concentrations of acetylcholine in the same brain area were not affected by repeated morphine administration (Week effect: F1, 4 = 0.01, P>0.92; Week × Time interaction F 5, 20 = 1.0, P>0.44).
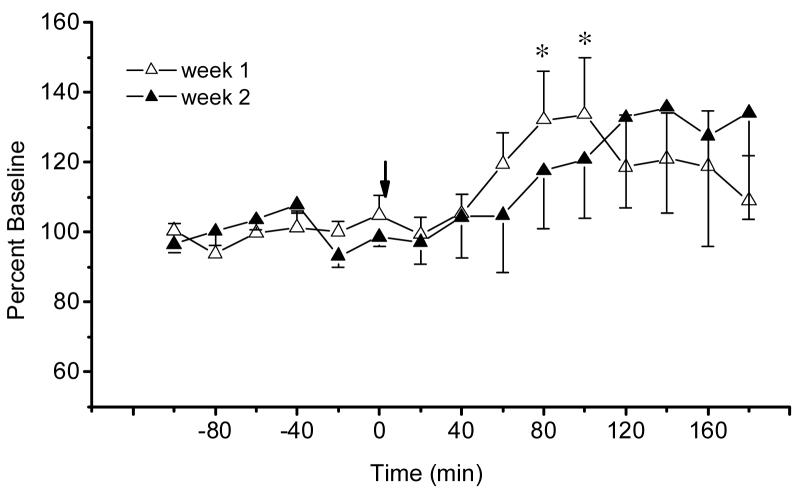
Consistent with the results from acute studies, the injection of morphine (5 mg/kg i.p.) during the first microdialysis session produced a significant increase of extracellular acetylcholine in the IPN of naïve rats (Time effect: F 14, 56 = 2.03, P<0.03). As shown in Fig.3, this increase was apparent at 80 and 100 min and reached its maximum of 133.6 % at 100 min after morphine administration. In morphine-experienced rats, the same dose of morphine had no significant effect on acetylcholine release in the IPN (Time effect: F 14, 56 = 1.02, P<0.45). Although this suggests that tolerance occurred, there was no significant difference between the week 1 and week 2 responses (Week effect: F1, 4 = 0.02, P>0.91; Week × Time interaction F 14, 56 = 0.71, P>0.76). Therefore, repeated administration of morphine produced neither tolerance nor sensitization to its enhancing effects on acetylcholine release in the IPN.
Figure 3.
Time-course of extracellular acetylcholine (mean ± S.E.M.) in the interpeduncular nucleus of rats treated with repeated morphine ( 5 mg/kg, i.p., n=5). Arrows indicate injections of morphine. *, P<0.05 treatment vs baseline, Fisher LSD tests.
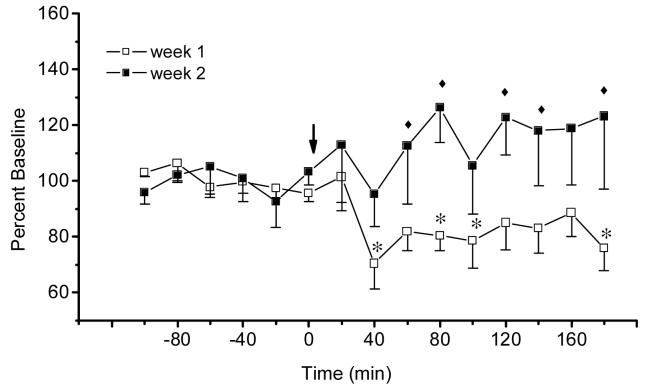
Intraperitoneal injection of morphine (20 mg/kg) to naïve rats produced a significant decrease of extracellular acetylcholine in the IPN of morphine-naïve animals, but not in morphine-experienced rats (for week 1, Time effect: F 14, 56 = 2.66, P<0.005; for week 2, Time effect: F 14, 56 = 0.61, P>0.84). As further analysis revealed, morphine-induced decrease of acetylcholine release during week 1 dialysis was significant at 40, 80, 100 and 180 min and reached its lowest value of 70.41 % of baseline at 40 min after treatment. Thus, consistent with our previous results, the first injection of morphine produced a significant decrease of extracellular acetylcholine in the IPN of naïve rats. Tolerance developed to this effect of morphine after repeated administration of morphine. Analysis across weeks supports this conclusion (Week effect: F1, 4 = 15.35, P<0.02). As shown in Fig.4, tolerance was apparent at all time points from 60 to 80 and 120-140 min as well as at 180 min after the injection.
Figure 4.
Time-course of extracellular acetylcholine (mean ± S.E.M.) in the interpeduncular nucleus of rats treated with repeated morphine ( 20 mg/kg, i.p., n=5). Arrows indicate injections of morphine. *, P<0.05 treatment vs baseline; ◆, P<0.05 week 1 vs week 2, Fisher LSD tests.
3.2 Behavioral studies
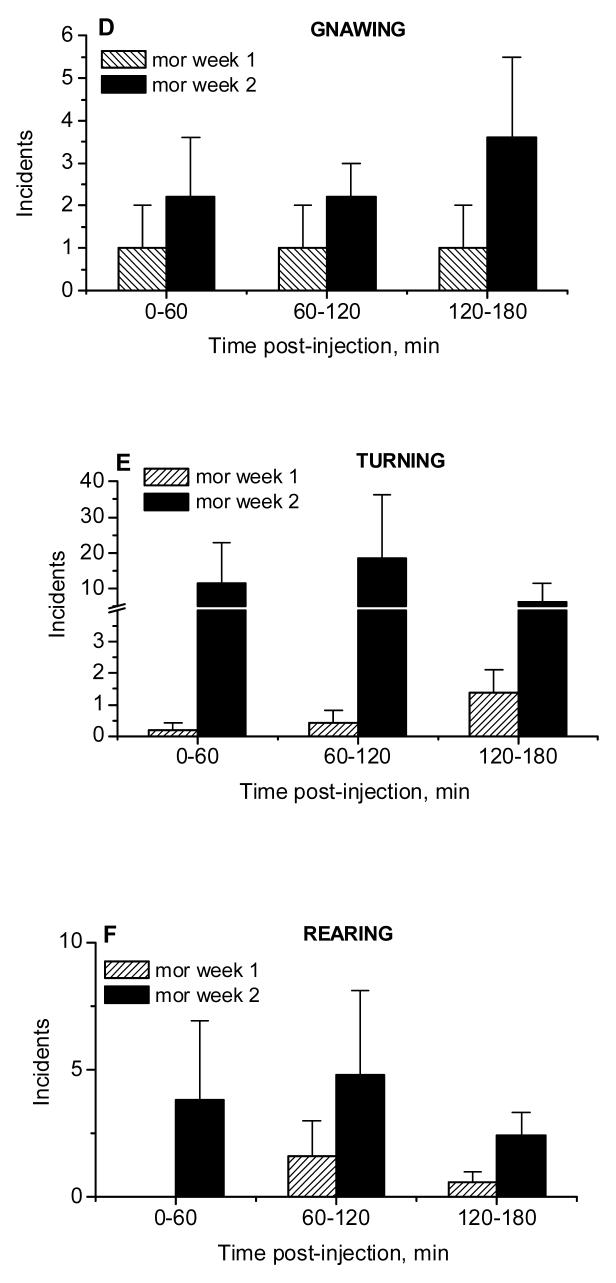
3.2.1 Administration of Morphine, 5 mg/kg (Fig.5)
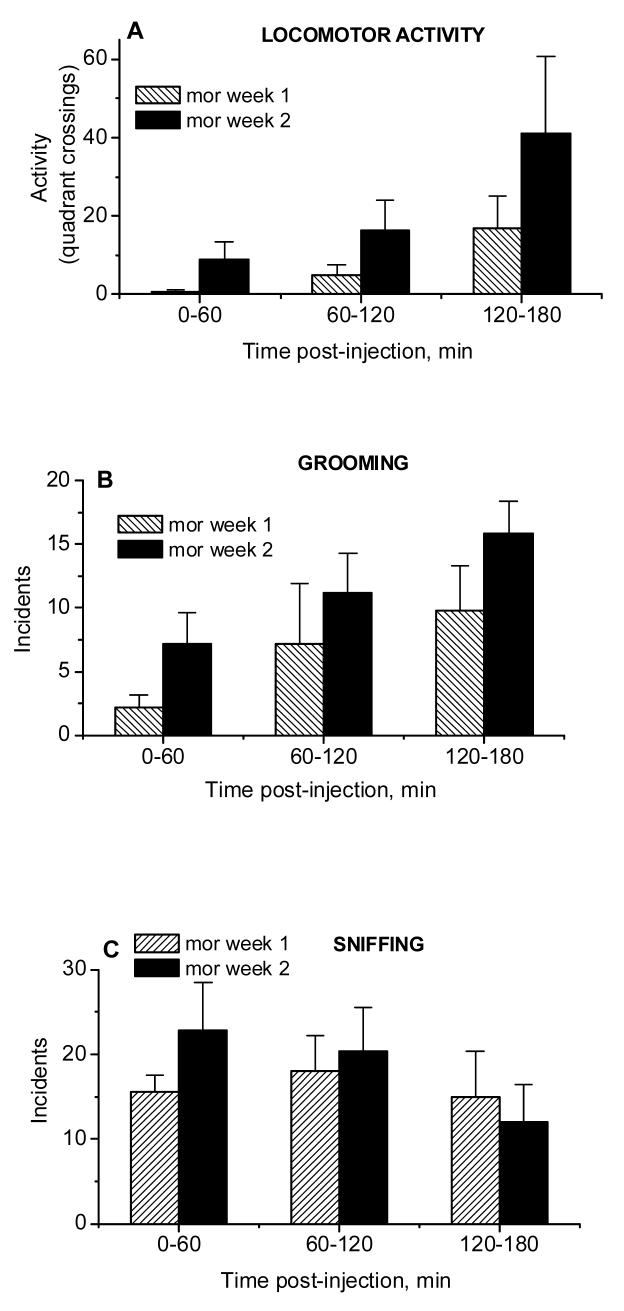
Figure 5 (A-F).
Locomotor activity (quadrant crossings, A) and incidents of grooming (B), sniffing (C), gnawing (D), turning (E) and rearing (F) assessed during microdialysis sessions in rats repeatedly treated with morphine (5 mg/kg, i.p., mean ± S.E.M., n=5). *, P<0.05 week 1 vs week 2, Fisher LSD tests.
To determine whether morphine-induced neurochemical changes in the habenulo-interpeduncular pathway of rats repeatedly treated with morphine are related to animal's motor behavior, the latter was assessed simultaneously with microdialysis sessions. The counts of locomotor activity in rats injected with saline were as follows: 0.80 ± 0.34, 0.87 ± 0.42, 0.80 ± 0.37 during the first, second and third hour of observation, respectively (mean ± SEM, n=5). The other behaviors similarly assessed in the same animals included grooming: 11.80 ± 3.76, 3.20 ± 0.97, 5.80 ± 2.76; sniffing: 0.80 ± 0.58, 0.40 ± 0.40, 1.60 ± 0.93; gnawing: 1.80 ± 1.56, 0.80 ± 0.58, 1.20 ± 1.20; turning: 0.20 ± 0.20, 0.60 ± 0.40, 0.60 ± 0.60 and rearing: 0.80 ± 0.37, 1.40 ± 0.87, 0.40 ± 0.40. Analysis of responses to acute morphine, compared to saline (data not shown), using repeated measures ANOVA with treatment as the main factor and time as repeated measure variable revealed that acute morphine increased locomotor activity in rats during microdialysis; this effect was apparent at 120-180 min after injection (Group × Time interaction: F 2,16 =4.07, P<0.04; post-hocs). Further analysis of rats' behavior revealed that acute morphine significantly decreased grooming; this effect was apparent during the first hour after injection (Group × Time interaction: F 2,16 = 4.44, P<0.03; post hocs). The same treatment also increased sniffing during the entire time of observation (Group effect: F 1,8 =26.52, P<0.01; Group × Time interaction: F 2,16 =0.35, P>0.71; post hocs). Acute morphine did not have an effect on incidents of gnawing, turning or rearing in naïve rats (for gnawing: Group effect: F 1,8 =0.03, P>0.86; Group × Time interaction: F 2,16 =0.88, P>0.43; for turning: Group effect: F 1,8 =0.14, P>0.72; Group × Time interaction: F 2,16 =1.29, P>0.30; for rearing: Group effect: F 1,8 =0.04, P>0.85; Group × Time interaction: F 2,16 =0.39, P>0.68).
The time-course of locomotor responses to morphine (5mg/kg i.p.) in naïve rats and those with morphine experience are shown in Fig.5A. The repeated administration of morphine did not alter its effects on locomotor activity (Week effect: F 1,8 =1.82, P>0.21; Week × Time interaction: F 2,16 =0.73, P>0.50). Likewise, the same treatment did not alter incidents of grooming, sniffing, gnawing, turning and rearing in morphine-experienced rats (Fig. 5 B-F; for grooming: Week effect: F 1,8 =1.86, P>0.21; Week × Time interaction: F 2,16 =0.12, P>0.89; for sniffing: Week effect: F 1,8 =0.20, P>0.66; Week × Time interaction: F 2,16 =0.88, P>0.43; for gnawing: Week effect: F 1,8 =1.01, P>0.34; Week × Time interaction: F 2,16 =1.18, P>0.33; for turning: Week effect: F 1,8 =1.0, P>0.35; Week × Time interaction: F 2,16 =1.08, P>0.36; for rearing: Week effect: F 1,8 =1.49, P>0.26; Week × Time interaction: F 2,16 =0.35, P>0.71). These results suggest that repeated administration of low doses of morphine did not induce behavioral tolerance or sensitization in rats.
3.2.2 Administration of morphine, 20 mg/kg (Fig.6)
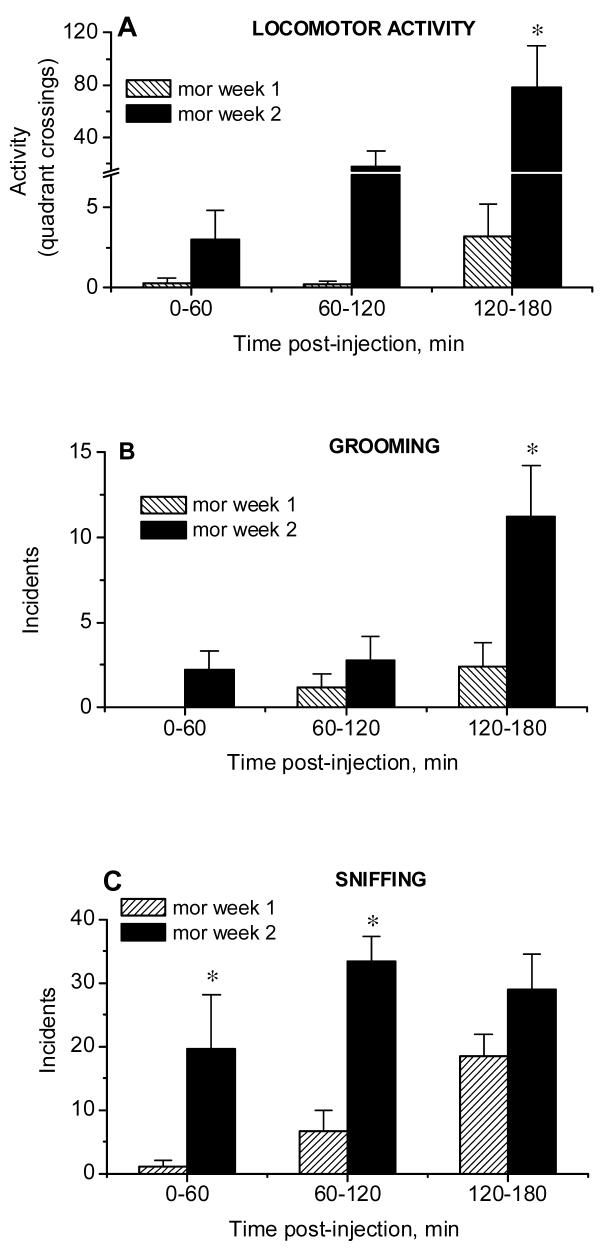
Figure 6 (A-F).
Locomotor activity (quadrant crossings, A) and incidents of grooming (B), sniffing (C), gnawing (D), turning (E) and rearing (F) assessed during microdialysis sessions in rats repeatedly treated with morphine (20 mg/kg, i.p., mean ± S.E.M., n=5). *, P<0.05 week 1 vs week 2, Fisher LSD tests.
Analysis of responses to acute morphine, compared to saline (data not shown), using repeated measures ANOVA with treatment as the main factor and time as a repeated measure, revealed no effect of treatment on locomotor activity of rats during the dialysis (Group effect: F 1,8 =0.33, P>0.58; Group × Time interaction: F 2,16 =1.99, P>0.17). Further analysis showed that while acute morphine significantly attenuated grooming during the first 60 min after the injection, the same treatment also increased the incidence of sniffing and gnawing (for grooming: Group effect: F 1,8 =24.45, P<0.001; Group × Time interaction: F 2,16 =2.65, P>0.101; for sniffing: Group effect: F 1,8 =16.96, P<0.003; Group × Time interaction: F 2,16 =9.42, P<0.002; for gnawing: Group effect: F 1,8 =2.58, P>0.15; Group × Time interaction: F 2,16 =4.74, P<0.02). The latter effects were significant during the last two hours and the last one hour of observation, respectively.
As shown in Fig.6A, the repeated administration of morphine increased the locomotor stimulant effects of the drug; this effect was apparent during the last 60 min of observation (Week effect: F 1,8 =4.78, P>0.60; Week × Time interaction: F 2,16 =5.76, P<0.013; post hocs). Furthermore, the repeated administration of morphine induced sensitization of all observed behaviors, except for rearing (for grooming: Week effect: F 1,8 =8.14, P<0.02; Week × Time interaction: F 2,16 =3.98, P<0.04; for sniffing: Week effect: F 1,8 =18.16, P<0.003; Week × Time interaction: F 2,16 =1.57, P>0.24; for gnawing: Week effect: F 1,8 =5.72, P<0.07; Week × Time interaction: F 2,16 =2.54, P>0.11; for turning: Week effect: F 1,8 =5.17, P<0.05; Week × Time interaction: F 2,16 =4.30, P<0.03; for rearing: Week effect: F 1,8 =3.49, P>0.10; Week × Time interaction: F 2,16 =1.43, P>0.27). Specifically, sensitization of grooming was apparent during the last 60 min of observation while sensitization of sniffing was significant during the first 120 min after the injection. Sensitization of gnawing and turning were observed from 60 to 120 and from 120 to 180 min after morphine, respectively. Thus, morphine-experienced rats displayed sensitization of locomotor and stereotypical responses to morphine.
4. Discussion
In the present study, for the first time, levels of extracellular acetylcholine in the interpeduncular nucleus were assessed in freely-moving rats. Previous studies have shown that the IPN has high concentrations of acetylcholine as well as choline acetyltransferase and acetylcholinesterase, suggesting an active turnover of acetylcholine in this nucleus (Contestabile and Fonnum, 1983;Sastry et al., 1979). Cholinergic projections to the interpeduncular nucleus come primarily from the ventral medial habenula (Contestabile and Fonnum, 1983). The habenulo-interpeduncular pathway is often referred to as “one of the major cholinergic pathways in the brain”(Morley, 1986).
Our results indicate that levels of extracellular acetylcholine in the interpeduncular nucleus can be altered by behaviorally relevant doses of morphine. A low dose of morphine (5 mg/kg, i.p.) enhanced the release of acetylcholine in the interpeduncular nucleus. It could be speculated that this effect of morphine was mediated via an indirect action on cholinergic cell bodies in the medial habenula or their terminals in the interpeduncular nucleus. Brain levels of morphine assessed with microdialysis after the same dose of morphine were reported to be approximately 3 nM (Matos et al., 1992), suggesting that activation of μ-opioid receptor signaling would likely occur (Christoffers et al., 2003). Mu-opioid receptors are densely expressed in the medial habenula and interpeduncular nucleus; however, their subcellular localization has not been described (Ding et al., 1996). It is conceivable that morphine decreased GABA release from forebrain projections to the medial habenula and thereby disinhibited acetylcholine-containing cells (Sutherland, 1982;Contestabile and Fonnum, 1983). Similar GABA-mediated disinhibition of septal cholinergic neurons via μ-opioid receptors was previously reported in the hippocampus (Alreja et al., 2000). Analogously, in the mesolimbic pathway, disinhibition of dopamine-containing cells is known to occur via morphine's action on GABA-ergic interneurons in the ventral tegmental area (Williams et al., 2001). Morphine could also act on GABA-ergic forebrain projections to the interpeduncular nucleus, causing a local decrease of GABA release and depolarization of cholinergic terminals (Dinopoulos et al., 1989). The same dose of morphine (5 mg/kg i.p.) has also been shown to increase extracellular acetylcholine levels in the rostral ventrolateral medulla (Taguchi et al., 1999).
The highest dose of morphine (20 mg/kg i.p.) produced a decrease in extracellular acetylcholine levels. At this dose morphine may activate both high affinity μ-opioid receptor and low affinity k-opioid receptors. Although some kappa-opioid binding has been identified in the medial habenula, kappa-binding is more densely distributed in the interpeduncular nucleus (Unterwald et al., 1991). Thus, it could be speculated that both k-opioid receptors on the cholinergic cells and previously discussed μ-opioid receptors on GABA-ergic terminals were jointly activated in the habenulo-interpeduncular pathway by the high dose of morphine. The observed decrease in acetylcholine release suggests that the kappa action prevailed. The same dose of morphine (20 mg/kg, i.p.) has also been shown to decrease extracellular acetylcholine levels in the nucleus accumbens (Rada et al., 1991).
Previous microdialysis studies of morphine-induced changes in acetylcholine in morphine-experienced rats demonstrated decreased or increased effects of the drug depending on its route of administration, the injection protocol and the brain area (Rada et al., 1996;Imperato et al., 1996;Rada et al., 1991). In the present study, after repeated morphine administration, tolerance developed to the inhibitory effect of the higher dose of morphine but not clearly to the effect of a low dose of morphine. These results could possibly be attributed to differential adaptations of μ and κ-opioid receptors on cholinergic cell bodies in the medial habenula and GABA-ergic terminals in the IPN (Nestler, 2004). It is also conceivable, that chronic morphine, depending on the dose, differentially targets neurotransmitter systems (e.g., GABA) upstream from the IPN. Thus, their relative contribution in regulation of acetylcholine release in the IPN might vary with the dose of morphine.
Repeated administration of 20 mg/kg of morphine was previously shown to induced locomotor sensitization in rats (Szumlinski et al, 2000); this effect is believed to be mediated by enhanced dopaminergic neurotransmission in the mesolimbic system (Wise and Bozarth, 1987;Cadoni and Di Chiara, 1999). Although assessed differently in the present study, locomotor activity was shown to increase in morphine-experienced rats during the second week of dialysis. Considered together with neurochemical data, these results indicate that sensitization to morphine-induced changes in locomotor activity is accompanied by tolerance to its effects on the habenulo-interpeduncular pathway. On the other hand, the low dose of repeated morphine (i.e., 5 mg/kg i.p.) did not produce sensitization of locomotor activity during the dialysis, and as noted above, there was no clear evidence of tolerance or sensitization on acetylcholine release in the IPN of the same rats. It is intersting to note that both doses of morphine used in the present study are known to be analgesic. As measured in tail flick and hot plate assays, acute doses of morphine, 5 mg/kg or 20 mg/kg i.p., induced analgesia in rats (Tsuchiya et al., 2006;Michaluk et al., 1998). Repeated administration of either dose induced tolerance, in seven days for the low dose and in eight days for the higher dose (Tsuchiya et al., 2006;Michaluk et al., 1998).
While hyperactivity induced by chronic morphine has been related to enhanced dopamine release in the mesolimbic system, other motor behaviors assessed in the present study are thought to be mediated by the activation of the nigrostriatal pathway (Brann et al., 1983;Volpicelli et al., 1999) Attenuation of grooming and increased sniffing behaviors in rats injected with acute morphine (5 and 20 mg/kg) as well as increased gnawing (20 mg/kg) were consistent with previous reports (Patti et al., 2005;Walter and Kuschinsky, 1989). Sensitization of five of the six assessed stereotypic behaviors occurred in rats treated with high dose of chronic morphine while no sensitization occurred in the group receiving the low dose of morphine.
The habenulo-interpeduncular pathway has been shown to interact with the mesolimbic pathway (see Introduction). Owing to multiple anatomical connections between the two pathways, it was proposed that these systems could together mediate the reinforcing properties of addictive drugs (Ellison, 1994). Acetylcholine, one of the predominant neurotransmitters in the interpeduncular nucleus (Contestabile et al., 1987), may be a major determinant of those interactions. Consistent with this premise, nicotinic antagonists locally injected into the IPN attenuated morphine-induced sensitization of dopamine responses in the nucleus accumbens (Taraschenko et al, 2005)as well as the self-administration of morphine (Glick et al., 2006).Thus, in the present study, acetylcholine might have acted at nicotinic receptors (Perry et al., 2002) to alter efferent neurotransmission from the IPN to the median and dorsal raphe (Groenewegen et al., 1986). The raphe nuclei, via their projections to the ventral tegmental area (Phillipson, 1979), can influence the firing of dopamine-containing cells. Alternatively, IPN can communicate with the nucleus accumbens and can influence the release of dopamine there via sequential glutamatergic connections between the mediodorsal thalamus and the medial prefrontal cortex (Morley, 1986;Pennartz et al., 1994;Vertes, 2006)
The habenulo-interpeduncular pathway has also been shown to influence the activity of dopaminergic neurons in the striatum, this interaction has also been thought to involve acetylcholine. Specifically, interruption of impulse flow in the habenulo-interpeduncular pathway by local infusion of tetrodotoxin reduced choline content in both in the IPN and the striatum (Takashima et al., 1992). Thus, in our study, tolerance to cholinergic effects of morphine could enhance morphine-induced dopamine release in the striatum, expressed as sensitization of stereotypic behavior.
In summary, the development of tolerance after repeated administration of morphine (20 mg/kg) on cholinergic neurotransmission in the habenulo-interpeduncular pathway may be linked to sensitization of dopaminergic neurotransmission in the mesolimbic and nigrostriatal pathways. This relationship between a tolerant habenulo-interpeduncular pathway and sensitized dopaminergic pathways is consistent with the reciprocal inhibitory relationship demonstrated previously (Nishikawa et al., 1986).
Acknowledgements
This research was supported by NIDA Grant DA 016283
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alreja M, Shanabrough M, Liu W, Leranth C. Opioids suppress IPSCs in neurons of the rat medial septum/diagonal band of Broca: involvement of mu-opioid receptors and septohippocampal GABAergic neurons. J.Neurosci. 2000;20:1179–1189. doi: 10.1523/JNEUROSCI.20-03-01179.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blander A, Wise RA. Anatomical mapping of brain stimulation reward sites in the anterior hypothalamic area: special attention to the stria medullaris. Brain Res. 1989;483:12–16. doi: 10.1016/0006-8993(89)90029-2. [DOI] [PubMed] [Google Scholar]
- Bot G, Chahl LA. Induction of Fos-like immunoreactivity by opioids in guinea pig brain. Brain Res. 1996;731:45–56. doi: 10.1016/0006-8993(96)00457-x. [DOI] [PubMed] [Google Scholar]
- Brann MR, Hacker M, Finnerty M, Ellis J, Lenox RH, Ehrlich YH. Automated analysis of stereotypic behavior induced by psychomotor stimulants. Pharmacol.Biochem.Behav. 1983;19:57–62. doi: 10.1016/0091-3057(83)90312-x. [DOI] [PubMed] [Google Scholar]
- Cadoni C, Di Chiara G. Reciprocal changes in dopamine responsiveness in the nucleus accumbens shell and core and in the dorsal caudate-putamen in rats sensitized to morphine. Neuroscience. 1999;90:447–455. doi: 10.1016/s0306-4522(98)00466-7. [DOI] [PubMed] [Google Scholar]
- Christoffers KH, Li H, Keenan SM, Howells RD. Purification and mass spectrometric analysis of the mu opioid receptor. Brain Res.Mol.Brain Res. 2003;118:119–131. doi: 10.1016/j.molbrainres.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Contestabile A, Fonnum F. Cholinergic and GABAergic forebrain projections to the habenula and nucleus interpeduncularis: surgical and kainic acid lesions. Brain Res. 1983;275:287–297. doi: 10.1016/0006-8993(83)90989-7. [DOI] [PubMed] [Google Scholar]
- Contestabile A, Villani L, Fasolo A, Franzoni MF, Gribaudo L, Oktedalen O, Fonnum F. Topography of cholinergic and substance P pathways in the habenulo-interpeduncular system of the rat. An immunocytochemical and microchemical approach. Neuroscience. 1987;21:253–270. doi: 10.1016/0306-4522(87)90337-x. [DOI] [PubMed] [Google Scholar]
- Ding YQ, Kaneko T, Nomura S, Mizuno N. Immunohistochemical localization of mu-opioid receptors in the central nervous system of the rat. J.Comp Neurol. 1996;367:375–402. doi: 10.1002/(SICI)1096-9861(19960408)367:3<375::AID-CNE5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Dinopoulos A, Papadopoulos GC, Parnavelas JG, Antonopoulos J, Karamanlidis AN. Basal forebrain projections to the lower brain stem in the rat. Exp.Neurol. 1989;105:316–319. doi: 10.1016/0014-4886(89)90136-2. [DOI] [PubMed] [Google Scholar]
- Ellison G. Stimulant-induced psychosis, the dopamine theory of schizophrenia, and the habenula. Brain Res.Brain Res.Rev. 1994;19:223–239. doi: 10.1016/0165-0173(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Glick SD, Ramirez RL, Livi JM, Maisonneuve IM. 18-Methoxycoronaridine acts in the medial habenula and/or interpeduncular nucleus to decrease morphine self-administration in rats. Eur.J.Pharmacol. 2006;537:94–98. doi: 10.1016/j.ejphar.2006.03.045. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Ahlenius S, Haber SN, Kowall NW, Nauta WJ. Cytoarchitecture, fiber connections, and some histochemical aspects of the interpeduncular nucleus in the rat. J.Comp Neurol. 1986;249:65–102. doi: 10.1002/cne.902490107. [DOI] [PubMed] [Google Scholar]
- Imperato A, Obinu MC, Casu MA, Mascia MS, Carta G, Gessa GL. Chronic morphine increases hippocampal acetylcholine release: possible relevance in drug dependence. Eur.J.Pharmacol. 1996;302:21–26. doi: 10.1016/0014-2999(96)00047-7. [DOI] [PubMed] [Google Scholar]
- Maisonneuve IM, Glick SD. Attenuation of the reinforcing efficacy of morphine by 18-methoxycoronaridine. Eur.J.Pharmacol. 1999;383:15–21. doi: 10.1016/s0014-2999(99)00560-9. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Miller M, Jr., Dworkin SI, Smith JE, Porrino LJ. Alteration of local cerebral glucose utilization following intravenous administration of heroin in Fischer 344 rats. Brain Res. 1997;755:313–318. doi: 10.1016/s0006-8993(97)00114-5. [DOI] [PubMed] [Google Scholar]
- Matos FF, Rollema H, Basbaum AI. Simultaneous measurement of extracellular morphine and serotonin in brain tissue and CSF by microdialysis in awake rats. J.Neurochem. 1992;58:1773–1781. doi: 10.1111/j.1471-4159.1992.tb10053.x. [DOI] [PubMed] [Google Scholar]
- Michaluk J, Karolewicz B, Antkiewicz-Michaluk L, Vetulani J. Effects of various Ca2+ channel antagonists on morphine analgesia, tolerance and dependence, and on blood pressure in the rat. Eur.J.Pharmacol. 1998;352:189–197. doi: 10.1016/s0014-2999(98)00373-2. [DOI] [PubMed] [Google Scholar]
- Morley BJ. The interpeduncular nucleus. Int.Rev.Neurobiol. 1986;28:157–182. doi: 10.1016/s0074-7742(08)60108-7. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular mechanisms of drug addiction. Neuropharmacology. 2004;47(Suppl 1):24–32. doi: 10.1016/j.neuropharm.2004.06.031. [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Fage D, Scatton B. Evidence for, and nature of, the tonic inhibitory influence of habenulointerpeduncular pathways upon cerebral dopaminergic transmission in the rat. Brain Res. 1986;373:324–336. doi: 10.1016/0006-8993(86)90347-1. [DOI] [PubMed] [Google Scholar]
- Patti CL, Frussa-Filho R, Silva RH, Carvalho RC, Kameda SR, TakatsuColeman AL, Cunha JL, Abilio VC. Behavioral characterization of morphine effects on motor activity in mice. Pharmacol.Biochem.Behav. 2005;81:923–927. doi: 10.1016/j.pbb.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press Inc.; San Diego, California: 1986. [Google Scholar]
- Pennartz CM, Groenewegen HJ, Lopes da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog.Neurobiol. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Perry DC, Xiao Y, Nguyen HN, Musachio JL, Davila-Garcia MI, Kellar KJ. Measuring nicotinic receptors with characteristics of alpha4beta2, alpha3beta2 and alpha3beta4 subtypes in rat tissues by autoradiography. J.Neurochem. 2002;82:468–481. doi: 10.1046/j.1471-4159.2002.00951.x. [DOI] [PubMed] [Google Scholar]
- Phillipson OT. Afferent projections to the ventral tegmental area of Tsai and interfascicular nucleus: a horseradish peroxidase study in the rat. J.Comp Neurol. 1979;187:117–143. doi: 10.1002/cne.901870108. [DOI] [PubMed] [Google Scholar]
- Rada P, Mark GP, Pothos E, Hoebel BG. Systemic morphine simultaneously decreases extracellular acetylcholine and increases dopamine in the nucleus accumbens of freely moving rats. Neuropharmacology. 1991;30:1133–1136. doi: 10.1016/0028-3908(91)90145-2. [DOI] [PubMed] [Google Scholar]
- Rada PV, Mark GP, Taylor KM, Hoebel BG. Morphine and naloxone, i.p. or locally, affect extracellular acetylcholine in the accumbens and prefrontal cortex. Pharmacol.Biochem.Behav. 1996;53:809–816. doi: 10.1016/0091-3057(95)02078-0. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res.Brain Res.Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Sastry BR, Zialkowski SE, Hansen LM, Kavanagh JP, Evoy EM. Acetylcholine release in interpeduncular nucleus following the stimulation of habenula. Brain Res. 1979;164:334–337. doi: 10.1016/0006-8993(79)90032-5. [DOI] [PubMed] [Google Scholar]
- Stewart J, De Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol.Rev. 1984;91:251–268. [PubMed] [Google Scholar]
- Sutherland RJ. The dorsal diencephalic conduction system: a review of the anatomy and functions of the habenular complex. Neurosci.Biobehav.Rev. 1982;6:1–13. doi: 10.1016/0149-7634(82)90003-3. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Nakajima S. Self-stimulation of the habenular complex in the rat. J.Comp Physiol Psychol. 1981;95:781–791. doi: 10.1037/h0077833. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Maisonneuve IM, Glick SD. The potential anti-addictive agent, 18-methoxycoronaridine, blocks the sensitized locomotor and dopamine responses produced by repeated morphine treatment. Brain Res. 2000;864:13–23. doi: 10.1016/s0006-8993(00)02069-2. [DOI] [PubMed] [Google Scholar]
- Taguchi K, Kato M, Kikuta J, Abe K, Chikuma T, Utsunomiya I, Miyatake T. The effects of morphine-induced increases in extracellular acetylcholine levels in the rostral ventrolateral medulla of rat. J.Pharmacol.Exp.Ther. 1999;289:1539–1544. [PubMed] [Google Scholar]
- Takashima M, Takita M, Umino A, Nishikawa T, Mitsushio H, Takahashi K. Modulation of cerebral acetylcholine metabolism by the dorsal diencephalic conduction system in the rat. Neurochem.Int. 1992;20:583–589. doi: 10.1016/0197-0186(92)90038-s. [DOI] [PubMed] [Google Scholar]
- Taraschenko OD, Ramirez RL, Livi JM, Maisonneuve IM, Glick SD. 18-Methoxycoronaridine acts in the medial habenula to attenuate opioid reward and mesolimbic dopamine sensitization to morphine. Soc.Neurosci. 2005 [Google Scholar]
- Tsuchiya T, Takeuchi T, Hayashida K, Shimizu H, Ando K, Harada E. Milk-derived lactoferrin may block tolerance to morphine analgesia. Brain Res. 2006;1068:102–108. doi: 10.1016/j.brainres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Knapp C, Zukin RS. Neuroanatomical localization of kappa 1 and kappa 2 opioid receptors in rat and guinea pig brain. Brain Res. 1991;562:57–65. doi: 10.1016/0006-8993(91)91186-5. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;142:1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Volpicelli LA, Easterling KW, Kimmel HL, Holtzman SG. Sensitization to daily morphine injections in rats with unilateral lesions of the substantia nigra. Pharmacol.Biochem.Behav. 1999;64:487–493. doi: 10.1016/s0091-3057(99)00098-2. [DOI] [PubMed] [Google Scholar]
- Walter S, Kuschinsky K. Conditioning of morphine-induced locomotor activity and stereotyped behaviour in rats. J.Neural Transm.Gen.Sect. 1989;78:231–247. doi: 10.1007/BF01249232. [DOI] [PubMed] [Google Scholar]
- Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81:299–343. doi: 10.1152/physrev.2001.81.1.299. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol.Rev. 1987;94:469–492. [PubMed] [Google Scholar]