Abstract
Mania is the defining feature of Bipolar Disorder (BD). There has been limited progress in understanding the neurobiological underpinnings of BD mania and developing novel therapeutics, in part due to a paucity of relevant animal models with translational potential. Hyperactivity is a cardinal symptom of mania, traditionally measured in humans using observer-rated scales. Multivariate assessment of unconditioned locomotor behavior using the rat Behavioral Pattern Monitor (BPM) developed in our laboratory has shown that hyperactivity includes complex multifaceted behaviors. The BPM has been used to demonstrate differential effects of drugs on locomotor activity and exploratory behavior in rats. Studies of genetically engineered mice in a mouse BPM have confirmed its utility as a cross-species tool. In a “reverse-translational” approach to this work, we developed the human BPM to characterize motor activity in BD patients. Increased activity, object interactions, and altered locomotor patterns provide multidimensional phenotypes to model in the rodent BPM. This unique approach to modeling BD provides an opportunity to identify the neurobiology underlying BD mania and test novel antimanic agents.
Keywords: Bipolar Disorder, mania, locomotor activity, exploratory, rat, mouse, Behavioral Pattern Monitor, Open Field, hyperactivity
1. Modeling BD mania
Bipolar disorder (BD) is a severe psychiatric disorder that affects approximately 2-7% of the general population (Friedman et al. 2006). Mania is the defining feature of BD although patients can also manifest depressive symptoms. During the manic episodes of BD, patients exhibit impulsive behavior, hypersexuality, pressured speech, flight of ideas, and motor hyperactivity, which have been conceptualized as inhibitory failures of behavior and thought (Goodwin and Jamison 1990). These symptoms profoundly impair and disrupt social, occupational, and family life in individuals with BD (Post et al. 2003). Despite the prevalence of this disorder, surprisingly few studies (Franks et al. 1983, Gooding and Tallent 2001, Murphy et al. 1999), have attempted to develop adequate translational models to elucidate the neurobiology underlying BD. One approach to bridge the gap between animal research and clinical research in BD is to develop translational models that extend human studies to animal paradigms that examine analogous constructs. Alternatively, one can use a “reverse translational” approach where existing measurements of activity in animals can be adapted to study activity in patients with BD. In this review, the authors discuss the development of the human Behavioral Pattern Monitor (BPM), an analog of the rodent BPM and a method with which to sensitively quantify the characteristics of human hyperactive and exploratory behavior. We will particularly address the requirements that must be met for translatable applicability, and introduce a novel behavioral paradigm in humans that is designed to enhance the translational potential of research on BD.
2. Multivariate assessment of Locomotor Behavior
Motor hyperactivity, which manifests as agitation, is a cardinal feature of the mania of BD and is typically quantified using observer-rated and self-report scales. Such scales may not be sensitive to potentially subtle alterations in activity levels and activity patterns. Moreover, they may not be informative about qualitative aspects of hyperactivity such as instances of stereotypy, nor differentiate between large-scale hyperactive movements (e.g. pacing around a room) and smaller-scale hyperactive behavior (e.g. fidgeting and restlessness). The current situation regarding the assessment of hyperactivity in BD individuals is not unlike the state of the art in assessing animal motor behavior forty years ago (Lat 1965). Since that time, several research groups (Eilam and Golani 1989, Geyer et al. 1982, 1986, Szechtman et al. 1985) have developed sophisticated approaches to quantify locomotor and exploratory behavior in rodents. The results from these approaches have shown that assessment of motor activity is complex (Eilam and Golani 1989, File and Wardill 1975, Geyer 1990, Geyer and Paulus 1996, Geyer et al. 1986, Paulus and Geyer 1996, Szechtman et al. 1985) but important for understanding a wide range of phenomena ranging from how drugs affect behavior to the phenotype of genetic mutant mice. We submit in this review that multivariate assessments of both the amounts and sequential patterns of motor actions in humans can potentially provide far greater knowledge on the characteristics of locomotor behavior as well as the effects of treatment compared to existing methods.
3. The Complex Conceptual Basis of Locomotor Behavior
The assessment of unconditioned locomotor and exploratory behavior has become one of the most widely used behavioral paradigms in rodents to determine the effects of various experimental manipulations, ranging from genetic changes, e.g. knockout mice, to pharmacological challenges, e.g. amphetamine-induced locomotor activity (Crawley 1999). This wide range of applications is based on the fact that unconditioned motor activity probes a variety of behaviors, can be recorded automatically, and can quickly generate an effect profile (Geyer 1990). A variety of different concepts have been applied to the interpretation of aspects of unconditioned motor behavior of rodents in an open field, including arousal, novelty seeking, diversive and inspective exploration, anxiety, stereotypy, and perseveration (Eilam and Golani 1989, Geyer 1990, Geyer et al. 1986, Golani et al. 1993, Lat 1965, Paulus and Geyer 1991, Sanberg et al. 1985, Szechtman et al. 1985). In an extensive review of the open field test, Walsh and Cummins suggest the open field elicits affective components of behavior such as fear and excitability (Walsh and Cummins 1976). Numerous investigators have recognized the necessity for analyses of multivariate profiles and/or spatio-temporal patterns of motor activity and proposed different approaches to quantify the various components of open field behavior (Drai and Golani 2001, Eilam and Shefer 1997, Ossenkopp et al. 1996, Robbins 1979). Some of these approaches were based on observer ratings, while others have attempted to automate the entire measurement process. As detailed below, the measurement approaches developed in studies in rats are now being applied to phenotypic assessments of mice.
Over the past three decades, our group has focused on two strategies in order to address several shortcomings of the traditional approaches to the characterization of unconditioned motor activity and exploratory behavior. First, we have developed multivariate assessment techniques, as have many other groups (Ossenkopp et al. 1996). Second, we have developed a range of novel quantitative measures based on nonlinear dynamical systems methods and fractal geometry to assess important aspects of the hierarchical and sequential organization of behavior. Here, we will describe our applications of these strategies first to the characterization and differentiation of the behavioral profiles produced in rodents by the administration of various psychostimulant drugs. Psychostimulants provide a relevant focus here because many animal models related to BD and especially to mania rely on the behavioral effects of these drugs. We will then illustrate how we have applied these same strategies and measures to the study of exploratory motor activity in hospitalized patients with BD and other psychiatric disorders.
4. Assessing Multivariate Behavioral Profiles
Although drugs such as amphetamine, phencyclidine, caffeine, nicotine, and MDMA have been characterized primarily as psychostimulants based upon how they affect locomotor activity, they have diverse mechanisms of actions and differential effects in a variety of behavioral paradigms. Because this classification represents an oversimplification, many investigators have developed additional tests and measures to help differentiate the behavioral profiles of these and other psychoactive drugs. As a consequence, the original Open Field was expanded to include computer-monitored activity chambers based on photobeams or video-tracking as well as measures of exploratory behaviors such as rearings or holepokes in holeboard chambers (Berlyne 1966, File and Wardill 1975, Geyer 1990, Geyer and Paulus 1996, Makanjuola et al. 1977, Paulus and Geyer 1996). Among others, we developed a computer-monitored activity chamber and called it the rat Behavioral Pattern Monitor (BPM). The BPM is a 30.5 by 61 cm chamber equipped with rearing touchplates on the walls and 10 holes in the floors and walls that serve as discrete stimuli for rodents to investigate. This system collects information about the locomotor movements and investigatory responses (rearings and holepokes) of rats at high levels of temporal and spatial resolution (Geyer et al. 1986). Our initial uses of the multivariate profiles of locomotor and investigatory behaviors provided by the BPM helped us elucidate the behavioral characteristics and neuropharmacological mechanisms of psychoactive drugs (Adams and Geyer 1982, Flicker and Geyer 1982).
Multivariate profiles have many advantages over univariate assessments that are limited to measures of the amount of activity. These advantages are most clearly demonstrated in research assessing the effects of stimulant drugs on rodents. Depending upon dose, drugs such as amphetamine, apomorphine, caffeine, 3-4-methylenedioxymethamphetamine (MDMA), nicotine, phencyclidine and scopolamine all induce similar increases in the amount of activity, as measured in the Open Field, photobeam activity chambers, or the BPM (Bushnell 1987, Collins et al. 1979, Fink and Smith 1979, Fitzgerald et al. 1988, Geyer et al. 1986, Gold et al. 1988, Gould et al. 2001, Krebs-Thompson et al. 1998, Kulkarni and Dandiya 1975, Meliska and Loke 1984, Paulus and Geyer 1992, Sessions et al. 1980). Some differential effects have been noted however, when even the simplest of multivariate assessments is used (see Table 1 for summary). For example, while caffeine also increased rearing behavior in the Open Field and BPM, as well as holepoking in the holeboard and BPM (Collins et al. 1979, Geyer et al. 1986, Meliska and Loke 1984, Rao et al. 1999), nicotine had no effect on rearing behavior in the Open Field or BPM (Geyer et al. 1986, Meliska and Loke 1984). In fact, higher doses of nicotine reduced the amounts of locomotion and holepoking behavior (Marco et al. 2005, Marks et al. 1986), likely due to hypothermic effects. Likewise phencyclidine can raise or lower exploratory levels dependent upon dose (Krebs-Thompson et al. 1998). Moreover, while the dopamine releaser amphetamine stimulates exploratory behavior in the holeboard apparatus and BPM, the direct dopamine agonist apomorphine inhibits holepoking behavior (Geyer et al. 1986, Makanjuola et al. 1977). Similarly, MDMA, a derivative of amphetamine that preferentially releases presynaptic serotonin rather than dopamine, also reduces holepoking and rearing behavior (Gold et al. 1988). Also, while stereotypy behavior is observed in conjunction with both amphetamine- and apomorphine-induced hyperactivity (Antoniou and Kafetzopoulos 1991, Geyer et al. 1986, Gordon and Beck 1984, Rebec and Bashore 1984), higher doses are required for amphetamine-induced stereotypy (Makanjuola et al. 1977). Additionally, the type of stereotypy induced by these various psychostimulants differs greatly, with amphetamine at low doses simply producing an exaggerated wide range of activities, while apomorphine produced a more restricted behavioral repertoire (Antoniou and Kafetzopoulos 1991, Geyer et al. 1987), and MDMA produced repetitive behaviors that could not be readily scored using the rating scales defined for amphetamine-treated animals (Gold et al. 1988). These differential effects highlight the need for multivariate assessment of activity, where fine discriminations can be made as to the distinctive characteristics of the behavioral profiles and thus the potentially unique effects of the compounds. We will return to the differentiation of the differing behavioral profiles of psychostimulants below, after describing additional ways to characterize sequential patterns of motor behavior.
Table 1.
Differential effects of stimulants on behavioral organization
| ‘Stimulant’ | Locomotor Activity |
Spatial d | Entropy h |
Spatial CV |
Temporal CV |
Exploratory Behaviors |
|---|---|---|---|---|---|---|
| Amphetamine | ↑ | ↓ ↔a ↑ | ↑ | ↓ | ↓ | ↔ a ↑ |
| Apomorphine | ↓ a ↑ | ↓ | n/a | ↑ | ↔b ↓ | ↓ |
| MDMA | ↑ | ↓ | ↓ ↑ | ↑ | ↔b ↓ | ↓ |
| Phencyclidine | ↑ | ↑ | n/a | ↑ | ↓ | ↓ a ↑ |
| Scopolamine | ↑ | ↓ | n/a | ↑ | ↔b ↓ | ↔ b ↑ |
| Caffeine | ↔b ↑ | ↔ | n/a | ↔ | ↔b ↓ | ↔ a ↑ |
| Nicotine | ↔b ↑ | ↔ | n/a | ↔ | ↔b ↓ | ↔ a ↓ |
denotes a dose-dependent change in effect
denotes a time-dependent change in effect
n/a denotes not assessed
5. Assessing the Organization of Unconditioned Exploratory Behavior
Despite the great advantages of multivariate assessments, they commonly retain the traditional approach of characterizing behavior in terms of pre-defined response categories. Psychomotor stimulant drugs, however, are known to disrupt normal behavioral responses, fragment behavioral sequences, and introduce new elements into the normal repertoire. The situation is complicated even further by the fact that rating scales designed for one drug (e.g. amphetamine) are often inappropriate for another drug, even one having a related chemical structure such as MDMA (Gold et al. 1988). This fundamental problem constrains the ability of traditional measures to support inferences about the relationship of drug effects to the normal behavioral repertoire of the animal. Second, the traditional approaches are insufficient in quantifying the sequential arrangement of behavioral elements. Third, in traditional approaches, the temporal and spatial resolution used to define the measures is chosen arbitrarily. This choice is based frequently on the qualitative separation of temporal and spatial scales. As our studies indicate, however, there appears to be no distinct separation of temporal scales. Instead, “pauses” and “behavioral actions” are found on all time scales. Moreover, this separation fundamentally neglects the hierarchical nature of behavioral organization.
Recent studies in rodents have clearly demonstrated the additional utility of assessments of sequential patterns of locomotor activity in pharmacological and neurobiological studies (Drai and Golani 2001, Eilam and Golani 1988, Geyer and Paulus 1996, Kafkafi et al. 2003), insofar as measures of the organization of the behavior provide further information that complements traditional multivariate profiles. Behavioral organization within this context can be defined as the selection, ordering, and sequencing of behavioral elements in response to external or internal stimuli to form flexible, yet stable, macroscopic patterns of behavior. The assessment of behavioral organization, as in the case with locomotor behavior, can be approached from both hierarchical and sequential points of view. Hierarchically, behavioral elements are thought to form organized behavioral components on successively larger spatial or temporal scales. Accordingly, the evaluation should quantify the scale-invariant properties of the behavioral organization. Sequentially, behavioral elements are thought to be arranged serially into organized behavioral patterns. Thus, the evaluation should quantify the sequence length-independent properties of the behavioral organization. Two of the measures described below were developed originally for studies of rat locomotor activity and are now being extended for studies of humans. These two measures, the spatial scaling exponent, d, and the dynamical entropy, h, quantify the hierarchical and sequential properties of behavioral organization, respectively. These measures have several advantages over traditional rating scales and other scale-dependent assessments of motor behavior. Specifically, this approach does not depend on response categories that are defined a priori, is resolution and sequence-length independent, can be extended to include time-dependent characteristics, and allows one to obtain detailed statistical evaluations.
Spatial scaling exponent, d, measures the hierarchical and geometric organization of behavior. Specifically, d is based on the principles of fractal geometry and describes the degree to which the path taken within an enclosure by the subject is one-dimensional or two-dimensional. To obtain spatial d, the distance traveled is plotted against the number of micro-events (defined as the smallest change that can be observed) using a double-logarithmic coordinate system, and a line of fit between these two variables is generated (Paulus et al. 1990, Ralph et al. 2001) . Spatial d typically varies between 1 (a straight line) and 2 (a filled plane), with values closer to 1 reflecting straight movements and values closer to 2 reflecting highly circumscribed, local movements. At both ends of this spectrum, the geometric pattern of movement around the BPM is highly predictable but exhibits either an almost straight-line movement or a highly circumscribed geometrical pattern, respectively. Exemplars of spatial d values for the locomotor paths exhibited by rodents and humans are presented in Figures 1-5. Whereas spatial d measures the hierarchical and geometric organization of behavior , the dynamical entropy h measures the degree to which behavior is observed along a continuum between complete order and disorder (Paulus et al. 1991). The derivation of h has been described in detail in our previous work (Paulus et al. 1990). Briefly, a given sequence of activity is compared to similar preceding sequences, and this comparison is conducted for varying sequence lengths. Lower values of h (low entropy) suggest highly predictable or ordered sequences of motor activity, while higher values (high entropy) suggest a greater variety, or disorder, in level of motor activity.
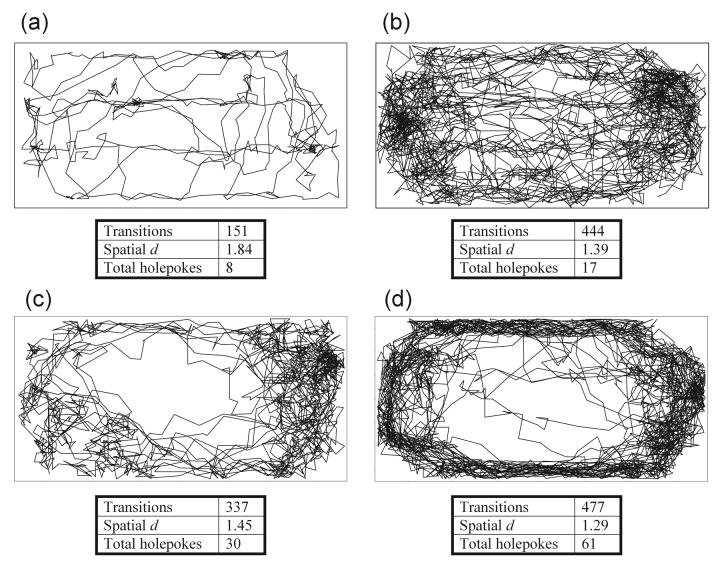
Figure 1. Stimulant effects on rat locomotor patterns in the Behavioral Pattern Monitor.
The effects of saline (a), amphetamine (b), phencyclidine (c) and scopolamine (d) on rat behavioral organization. a) The rat treated with saline explored little of the environment, making two or three excursions around the chamber, exhibiting short meandering movement in one or two areas then moving on. b) The amphetamine-treated rat however made numerous circuits of the chamber, crossing from one area to another in relatively straight lines, crossing the center as often as being close to the chamber walls, leading to a great variability of movement. The rat also exhibited more than one area of focused activity [‘home corner’ (Geyer 1982) or ‘home base’ (Eilam and Golani 1989)] c) The phencyclidine-treated rat exhibited sweeping movement patterns, from one corner to another, often circling from a home base that covered the right side of the chamber, with repetitive movements. d) The scopolamine-treated rat displayed increased long and straight movements particularly close to the chamber walls, deviating very little from this path. The level of activity was as great as amphetamine but with scopolamine the rat did not spend time focusing on any areas in particular, nor did it cross the center very often, displaying very repetitive movements. Data are provided for measures of locomotor activity (transitions), locomotor pattern (spatial d) and exploration (holepokes).
Figure 5. Locomotor patterns of human subjects in the human Behavioral Pattern Monitor.
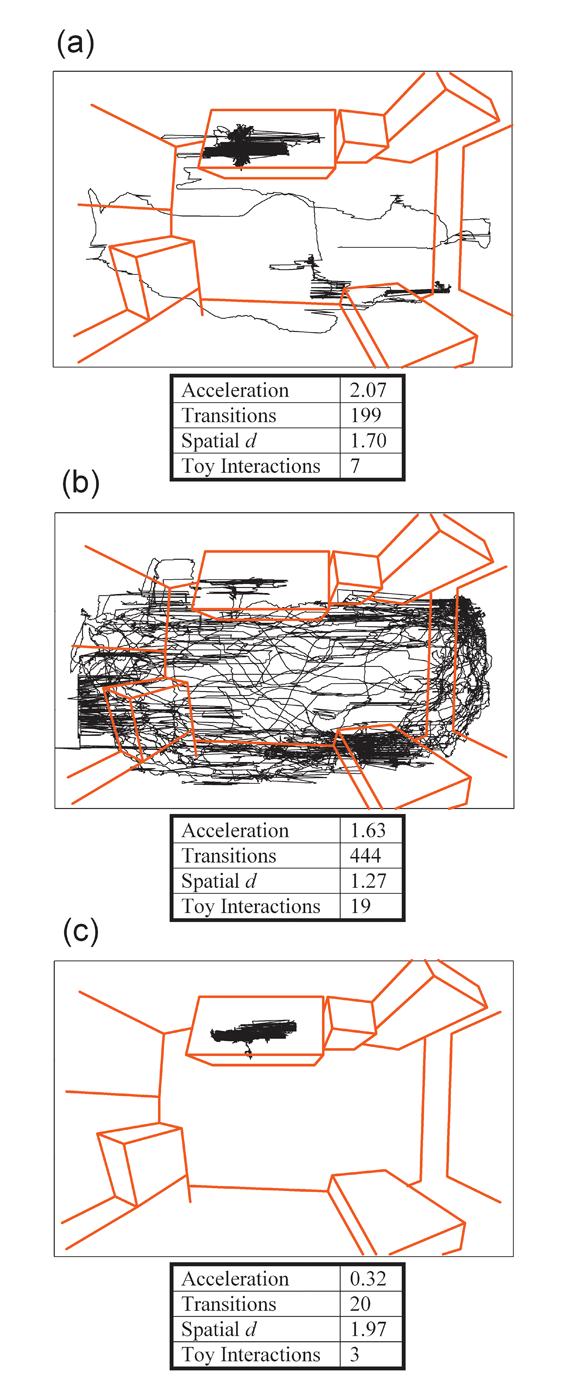
The layout of the room as observed through a fisheye lens is outlined in red. The locomotor pattern of a representative healthy subject (a), or manic BD (b) and schizophrenia (c) patients are shown in black. The location of the subject's upper torso (specifically, the LifeShirt vest) in x- and y-coordinates was recorded by tracking software (Clever Systems, Inc) as the subject examined the room and the objects located therein. An accelerometer embedded in a wearable ambulatory monitoring device (Lifeshirt) also recorded levels of motor activity in digital units for each subject. a) The healthy comparison subject walked around the room once, investigated the window, which is covered, examined some objects placed on the bookshelves farthest from the door, and finally moved to the desk, spending the remainder of time examining that area and the objects found there. b) The manic BD patient conducted numerous excursions around the room, often concentrating movement at specific locations such as the window and bookshelves farthest from the door and the small filing cabinet. Apart from the obvious quantity of movements that differentiate this subject from the healthy comparison subject, the manic BD subject also clearly failed to exhibit a preference for one location, spending time in numerous areas. This subject also displayed longer tracks of movement from one area of the room to another, with a large variability in the paths chosen. c) The schizophrenia patient displayed a virtual lack of exploratory behavior. This subject remained at the desk for the duration of the session. Some objects were investigated on the desk, but all exploration was specific and within a limited area. The quantitative data are shown for these representative subjects' acceleration, transitions, spatial d, and exploratory behavior (object interactions).
Another aspect of the spatial pattern of the animal's movement is described by the spatial CV statistic, which reflects the degree to which an animal makes the same repetitive transitions from one area to another and is described in greater detail elsewhere (Geyer et al. 1986). Analogously, the amount of time spent in each of the regions can also be quantified and used to calculate a CV statistic in the time domain. This temporal CV defines the degree to which the animal remains in one area or distributes its time across multiple areas (high vs. low temporal CV respectively; (Geyer et al. 1986). Collectively, such descriptors as spatial scaling exponent d, dynamical entropy h, spatial CV, and temporal CV assess various aspects of the hierarchical and sequential organization of behavior at a macroscopic level. This approach using non-linear measures has provided a useful complement to the more traditional behavioral profiles based on a priori definitions of categorical events (Paulus and Geyer 1991, Paulus and Geyer 1996).
In order to determine whether these approaches truly capture different behavioral variance, we conducted factor analyses on 137 control rats (Paulus and Geyer 1993b). The analyses revealed that the various automated assessments obtained from locomotor behavior in the BPM loaded onto three primary factors: the amount of activity (e.g. total photobeam breaks, transitions from one region of the BPM to a neighboring region, and distance traveled); exploratory behavior (e.g. rearing and holepoking); and sequential behavioral organization (spatial scaling exponent d, dynamical entropy h). Together, these three factors accounted for 77% of the variance in the measures. Subsequent studies have demonstrated that composite variables comprised of the weighted measures for each factor are highly effective in discriminating receptor subtype-specific drug effects in rats (Krebs-Thomson and Geyer 1998)
6. Combining Multivariate Profiles and Measures of Behavioral Organization
Using this approach, stimulants can be differentiated into those that do or do not affect exploratory behavior and/or behavioral organization (see Fig 1 comparing saline vs. amphetamine, phencyclidine, and scopolamine locomotor patterns). For example, apomorphine, MDMA, phencyclidine and scopolamine increase spatial CV, reflecting the generation of more repetitive patterns of movements, amphetamine increases the variety of different movements and therefore lowers spatial CV (Geyer et al. 1986, Gold et al. 1988, Lehman-Masten and Geyer, 1991). In contrast, neither nicotine nor caffeine had any effect on this measure. Appropriately, opposite effects are observed using the spatial d measure, where apomorphine and scopolamine lower spatial d, suggestive of movements in straighter lines, amphetamine increases this measure at low doses and produces both increases and decreases at high doses, phencyclidine only increases spatial d, while nicotine and caffeine do not affect spatial d (Geyer et al. 1986, Paulus and Geyer 1993a, Lehman-Masten and Geyer, 1991). Both amphetamine and MDMA increase entropy h, suggesting disordered movement, although higher doses of MDMA lower entropy h (Paulus et al. 1990). The only measure of behavioral organization that is affected similarly by all these psychostimulants is that of temporal CV. Amphetamine, apomorphine, scopolamine, nicotine, phencyclidine and caffeine all lower temporal CV, largely due to their common effect of generating hyperactivity that reduces the predominant behavior of the untreated rat to spend time in its home corner (Geyer et al. 1986, Lehman-Masten and Geyer, 1991). Thus despite these compounds all being categorized as psychostimulants, they produce distinctive behavioral profiles that can be readily differentiated using multivariate assessments including analyses of movement patterns.
This observation is hardly surprising given the different mechanisms of actions of each of these drugs. Neurochemical analysis can differentiate between these drugs and it is reassuring to know that behavioral analysis of spontaneous locomotion and exploration can also differentiate between them. Apomorphine and scopolamine share several characteristics, despite the former being an agonist at D1 and D2 dopamine receptors while the latter is a non-selective antagonist of the muscarinic acetylcholine receptors. While nicotine and caffeine share some characteristics, they differ in some aspects as well as their mechanism of action. Nicotine is the prototypical ligand of the nicotinic acetylcholine receptors, while caffeine may act via the GABAergic system, which can indirectly affect dopamine and norepinephrine functions. The myriad effects of amphetamine are a result of increased release of dopamine, serotonin, and norepinephrine, which occur via amphetamine-induced blockade of the respective transporters. Hence it is observed that other drug-induced manipulations of these neurotransmitter systems can also cause hyperactivity and altered exploratory behavior and locomotor patterns. With respect to modeling BD mania in animals, the literature is focused largely on the dopaminergic systems activated by drugs such as amphetamine. Perhaps future studies will examine the degree to which hyperactive behavioral profiles engendered by cholinergic, glutamatergic, or noradrenergic drugs – as well as dopaminergic drugs - may mimic the hyperactive states associated with mania in BD patients.
7. Locomotor Behavior Homologies: From Rat to Mice
The use of simple photobeam activity chambers (Young et al. 2007) or the open field test (Barr et al. 2004, Crusio 2001) has predominated in mouse locomotor research. We initially used a video-tracking system to assess motor activity in mice. As expected, psychostimulant drugs such as amphetamine produce similar degrees of activation in mice as in rats. Fairly dramatic differences in the patterns of activation produced by amphetamine in different strains of inbred and outbred mice were readily demonstrable (Ralph et al. 2001). Numerous studies have demonstrated the importance of the manipulation of the dopaminergic and serotonergic systems in altering locomotor activity and behavioral organization (Barr et al. 2004, Cabib et al. 2002, Grailhe et al. 1999, Powell et al. 2004). Moreover, a factor analysis on data collected from 84 mice demonstrated that, similarly to rats, transitions and distance traveled both load onto a factor of amount activity, while locomotor pattern measures such as the spatial scaling exponent d and dynamical entropy h load onto a behavioral organization factor (Paulus et al. 1999). The notable lack of exploratory behavior as an independent factor was likely determined by the video-tracking paradigm's inability to record rearing and holepoking behavior.
It has been suggested that the manic episodes in BD are consistent with a temporarily dysregulated dopamine system (Diehl and Gershon 1992, Emilien et al. 1999), reflecting exaggerated activation of brain dopamine systems. This suggested hyperdopaminergia is consistent with genetic linkage studies connecting dopamine transporter (DAT) abnormalities to BD (Greenwood et al. 2001, Hayden and Nurnberger 2006), especially as mice with reduced DAT levels exhibit significantly increased extracellular dopamine activity similarly to BD patients (Vawter et al. 2000, Zhuang et al. 2001). Moreover, amphetamine, which acts at the DAT, has been used widely in animal models of mania (Hayden and Nurnberger 2006). Hence, we initiated studies examining the activity of mice having complete or partial deletions of the gene for the DAT. Our initial studies of the DAT KO mouse using the video-based open field confirmed and extended previous observations of dramatic hyperactivity when these mutant mice are stimulated or placed in a novel environment (Gainetdinov et al, 2001). Although their activity levels are normal if undisturbed in their homecages, in a novel chamber, the locomotor activity of DAT null mutant mice is characterized by repetitive, perseverative straight movements in the periphery of the enclosure. In contrast to both DAT wildtype (WT) and heterozygous mice, these animals did not sample the entire enclosure; rather, the DAT KO showed a restricted repertoire of locomotor behavior (Ralph et al. 2001). We also found that DAT knockdown (KD) mice, lacking only 90% of the DAT, also exhibit a hyperactive phenotype characterized by more perseverative patterns of locomotor behavior reflected in lower spatial d values (Ralph-Williams et al. 2003). If indeed a dysregulated dopamine system underlies some of the key symptoms of mania, we hypothesized that pharmacological agents that successfully treat manic symptoms would attenuate the hyperactivity displayed by the DAT KD mutant mice. Indeed, when the DAT KD mice were treated with 100 mg/kg valproate, a dose that had no effect on WT mice, their hyperactivity was reduced significantly. Furthermore, while drug treatment had no effect on spatial d in the WT mice, valproate attenuated the perseverative patterns of motor behavior (diminished the predominance of straight sequences of locomotor activity as evidenced by increased spatial d) seen in the DAT KD mice. Thus, when the DAT KD mice were treated with the clinically effective antimanic drug valproate, both their hyperactivity and their perseverative motor behavior were significantly attenuated (Ralph-Williams et al. 2003).
Encouraged by such findings, we developed a mouse version of the BPM (Risbrough et al. 2006) designed to overcome the lack of exploratory behavior assessment in the video-based open field test. The utility of this mouse BPM was demonstrated in the description of the different locomotor activity, exploratory behavior, and behavioral organization phenotypes of dopamine receptor KO mice and their responses to MDMA (Risbrough et al. 2006). In another example of cross-species comparability, MDMA increased activity, lowered spatial d, and increased spatial CV in WT littermate mice in a similar pattern of responses to that observed in rats. The mouse BPM was also sufficiently sensitive to differentiate between the phenotypes of D1, D2, and D3 receptor KO mice. D1 KO mice exhibited an exaggerated responsiveness to the MDMA-induced increases in locomotor activity, while D2 KO mice exhibited a reduced amount of MDMA-induced activation (Risbrough et al 2006). Activity levels of D3 KO male mice were unaffected by MDMA while females displayed a reduced expression of MDMA-induced locomotor activation. Interestingly, D3 KO mice did not exhibit the same immediate MDMA induced-increase in perseverative locomotor behavior (spatial CV). While D1 KO mice exhibited straighter locomotor patterns (lower spatial d) than their WT counterparts, the spatial d measures of the D1 KO and WT mice were similarly lowered following MDMA administration (Risbrough et al. 2006). Hence it was suggested that D1 receptors may contribute to the locomotor pattern quality, i.e. linear vs. circumscribed movement (spatial d), while D2 receptors may contribute to perseverative or thigmotactic locomotor effects of MDMA (spatial CV). This study demonstrates the utility and cross-species generalizability of the BPM. Moreover, the mouse BPM data collected to date does suggest that a factor analysis could be performed similarly to that of rats, which would likely yield the emergence of exploratory behavior as a third independent factor.
The development of the BPM from rat to mouse is important for two reasons. First the cross-species generalization of the BPM is evident, as discussed above, with supporting evidence of similar locomotor pattern effects of amphetamine in both rats (Fig. 1) and mice (Fig. 2). Secondly, and perhaps more importantly, the development of the mouse BPM allows testing to be performed in genetically modified animals. Extending our previous work with DAT-deficient in the video-based open field, we have now begun using the mouse BPM in an extensive phenotyping of DAT KD mice. As expected, we have confirmed that in addition to their locomotor hyperactivity, they also exhibit increased exploratory behavior as measured by holepokes and reduced spatial d (Fig. 3). In fact when their locomotor pattern is plotted, it is evident that they display a similar profile to that of rats and mice administered low doses of amphetamine (Fig. 1b and 2b) and similarly to mice administered GBR12909 (Fig. 4), a selective DAT reuptake inhibitor. Moreover multiple ‘home bases’ are evident consistent with previous reports (Eilam and Golani 1990) that the ‘home corner’ described as characteristic of untreated rats (Geyer 1982) can elaborate into multiple preferred locations. Most importantly, the behavioral repertoire displayed by the DAT KD mice appeared similar to the behaviors that are often described of BD patients and recently observed by our group (see below). Thus assessing the ‘manic’ behavior of other putative models of BD mania in the mouse BPM may provide further support for these models, such as CLOCK mutants (McClung 2007, Naylor et al. 2000).
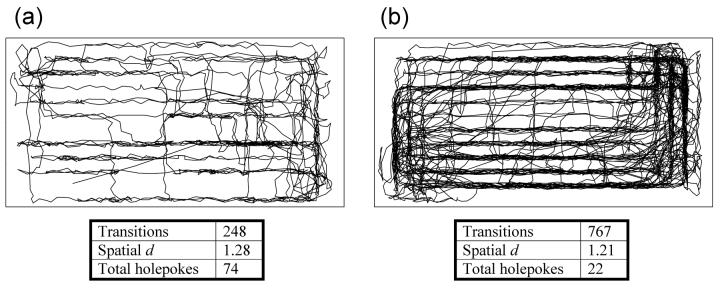
Figure 2. Effects of amphetamine on mouse locomotor patterns in the Behavioral Pattern Monitor.
The effects of saline (a) and amphetamine (b) on the behavioral organization of a representative mouse are presented. a) Mice treated with saline exhibit exploration throughout the chamber, but only perform a limited number of excursions around the chamber. This animal spent most of the time in the bottom right hand side of the chamber (the “home corner”). b) Mice treated with amphetamine however, exhibit a large number of excursions around the chamber, covering the chamber floor many times in a variety of paths. They also display several areas where their behavior is concentrated, suggesting several home bases as opposed to the one home corner observed in the saline administered mouse. While the x-y plots represented here are genuine movements, they also reflect a limitation of the number of photobeams used to identify a subject's position. Data are also provided for measures of locomotor activity (transitions), locomotor pattern (spatial d), and exploration (holepokes). Mice treated with amphetamine display a hyperactive phenotype, with lower spatial d and lower exploratory behavior when compared to control animals.
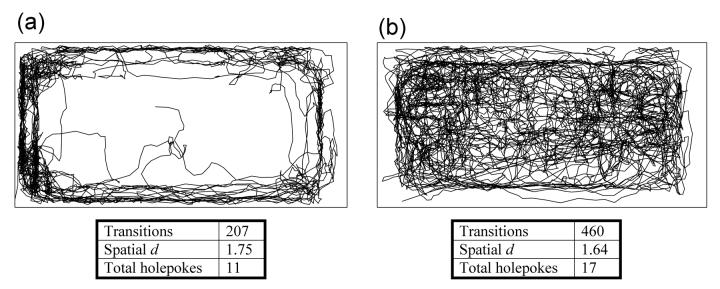
Figure 3. Locomotor patterns of dopamine transporter knockdown and wildtype littermate mice in the Behavioral Pattern Monitor.
Representative locomotor patterns of dopamine transporter wildtype (WT; a) and knockdown (KD; b) mice are shown. a) WT mice spend most of their time near the chamber walls and while some movement is made to explore the center, activity is concentrated in the left wall, where the mouse circles back and forth. b) In contrast to the time spent in the home corner by the WT mouse, this KD mouse displayed numerous areas of interest and exhibited more varied paths of activity. Locomotor activity (transitions), pattern (spatial d), and exploration (holepokes) data are provided, with the KD mice displaying greater activity, exploratory behavior, and straighter line movements (lower spatial d) when compared to their WT littermates.
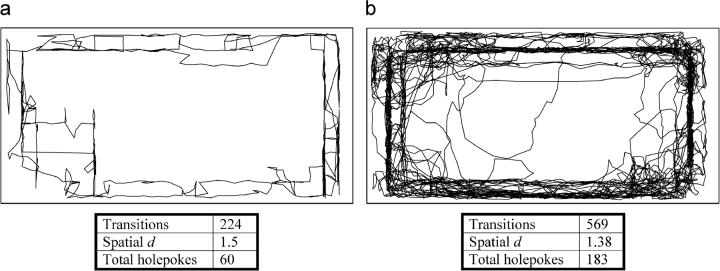
Figure 4. Effects of the selective dopamine transporter uptake inhibitor GBR 12909 on mouse locomotor patterns in the Behavioral Pattern Monitor.
The representative locomotor patterns are shown for mice treated with saline (a) or GBR 12909 (b). a) Mice treated with saline display very limited activity, making only one or two excursions around the chamber with limited exploration into the center of the chamber. b) Mice treated with GBR 12909 display far greater levels of activity, with numerous areas of focused activity and greater variety of paths taken. Data are also presented for locomotor activity (transitions), pattern (spatial d), and exploratory behavior (holepokes) with mice treated with GBR 12909 displaying hyperactivity, increased exploratory behavior, and straighter line movements (lower spatial d) than mice treated with saline.
8. The Human Behavioral Pattern Monitor
The richness of the results from locomotor behavior in rodents in terms of elucidating the underlying neural mechanisms has not been paralleled by similar studies in humans. Thus, to date a comparable laboratory-based, multivariate assessment of locomotor activity has not been extended to human populations. Some earlier studies have measured motor activity in psychiatric populations with the use of a wrist or leg accelerometer (Teicher 1995, Teicher et al. 1986, Wolff et al. 1985). The vast majority of studies on hyperactivity, however, have been limited to observer-rated and self-report scales. As discussed above, such scales may not be optimal in detecting potentially subtle alterations in activity levels nor are they informative about qualitative aspects of hyperactivity that may distinguish certain psychiatric populations from others. For example, several diagnostic groups may present with symptoms of hyperactivity but they may be qualitatively distinct and thus reflect different underlying neural circuitry abnormalities.
In response to the need for models of mania and following our work in rodents, we developed the human Behavioral Pattern Monitor(human BPM), as an analog of the rodent BPM and a method with which to sensitively quantify the characteristics of human hyperactive and exploratory behavior. Unlike other translational paradigms, the human BPM reflects a “reverse-translational” approach using the existing rich animal literature to inform its development. This approach contrasts with other well-established translational paradigms such as prepulse inhibition of the startle response, an index of sensorimotor gating. The assessment of prepulse inhibition in psychiatric patients was first developed in humans using the startle eyeblink response (Braff et al. 1995). It was subsequently extended to rats (Geyer et al. 2001) and then mice (Geyer et al. 2002), using the whole-body startle response. In contrast, the human BPM represents an evolution in the opposite direction, where a paradigm originally developed in rodents is mimicked in humans. The implementation of prepulse inhibition and the human BPM are examples of the bidirectionality of translational science, where human studies inform animal research, and vice-versa.
The human BPM takes place in a 9' by 14' room that the human participant has not been exposed to and therefore is, like the rodent BPM, a novel and unfamiliar environment. As detailed below, similar to the rodent BPM, multiple measures of motor activity can be collected, including spatial d, entropy h, transitions, distance traveled, and others. Along the walls of the room, dispersed evenly on items of furniture, are ten small objects. These objects were chosen using the criteria that they be safe, colorful, tactile, and manipulable and therefore invite human exploration. The objects provide an analog of the exploratory holes in the walls and floor of the rodent BPM chambers. Participants are directed into the room with little instruction or direction and are asked to wait for the experimenter to return. The human BPM session has been fifteen minutes long in our studies to date.
Data in the human BPM are gathered using three sources of measurement: 1) collection of physiologic data, namely motor activity of the subject's torso, using an accelerometer embedded in an ambulatory monitoring device that the participant wears; 2) x-y coordinates of the subject's spatial location in the BPM, extracted from digital video recording; and 3) experimenter ratings of exploratory activity, obtained by carefully scoring the video recording of the BPM session and measuring events such as interactions with objects. These three sources of measurement capture different qualitative aspects of motor and exploratory behavior, and yet may also be intercorrelated in the case of certain types of behavior, as will be illustrated below. In fact it is hypothesized that similarly to the rat and mouse BPM, three main independent factors will emerge, describing activity (accelerometry, transitions from one region to a neighboring region), exploratory behavior (interaction with objects), and sequential organization of behavior (spatial dand spatial CV).
In the human BPM, one measure of the amount of activity is quantified with an accelerometer, which is embedded in a wearable ambulatory monitoring device. The LifeShirt System (Vivometrics 2002) is an ambulatory, multi-sensor, continuous monitoring system that collects objective physiologic data through various sensors, including respiratory inductive plethysmography bands, which measure pulmonary function, electrical activity of the myocardium via a 3-lead EKG, and activity/posture via a two-axis accelerometer. The sensor array of the LifeShirt System is embedded in a sleeveless undergarment. For measurement of activity level, a two-axis accelerometer is placed onto the shirt over the sternum, and the rectified and integrated accelerometer signal is used to detect periods of physical activity and rest. An on-board PDA continuously encrypts and stores the patient's activity and postural physiologic data on a compact flash memory card. Accelerometry data are sampled at 10 Hz and stored numerically in digital units. Thus, one measure of the amount of motor activity is obtained by averaging acceleration values over the three five-minute intervals of the human BPM session. Exemplars of the acceleration values derived from individual subjects are provided in Figure 5.
To obtain additional measures of the quantity and patterns of motor activity and exploratory behavior, the room is also equipped with a camera and fish-eye lens system hidden in a ceiling vent. The images from the camera are stored in digital format on a computer in the adjacent room, with a frequency of 30 frames per second. The digital videos of subject's activity in the human BPM are subjected to frame-by-frame analysis with proprietary software (Clever Systems, Inc. 1999), which generates xand y-coordinates of the subject's successive locations. Because the software specifically tracks the blue LifeShirt vest, the coordinate positions reflect the position of the upper part of the subject's torso. At present, these x-y coordinates are used to plot the path of the subject and to count time spent and transitions between nine arbitrarily defined regions of the human BPM. These regions are analogous to our definition of nine areas of the rodent BPM (Geyer et al. 1986), namely the four corners, four walls, and the center. Delineation of these regions allows us to obtain a distribution of amount of time spent in each region as well as to measure the number of transitions, defined identically to the rodent work as movement from one region to an adjacent one. As we gain further experience with data generated using this system, alternative definitions of regions will no doubt be found to be more relevant to the behavior of humans in this environment than are the regions defined previously for use in rodents. In any event, transitions between regions and dwell times within specific regions can serve as additional measures to describe different aspects of locomotor activity and to complement the accelerometry data. In addition, the digitized video images enable detailed assessments of the subject's interactions with the 10 objects placed in the room, in analogy to the rodent's investigatory behavior directed toward the 10 holes placed in the walls and floors of the rodent BPM chambers.
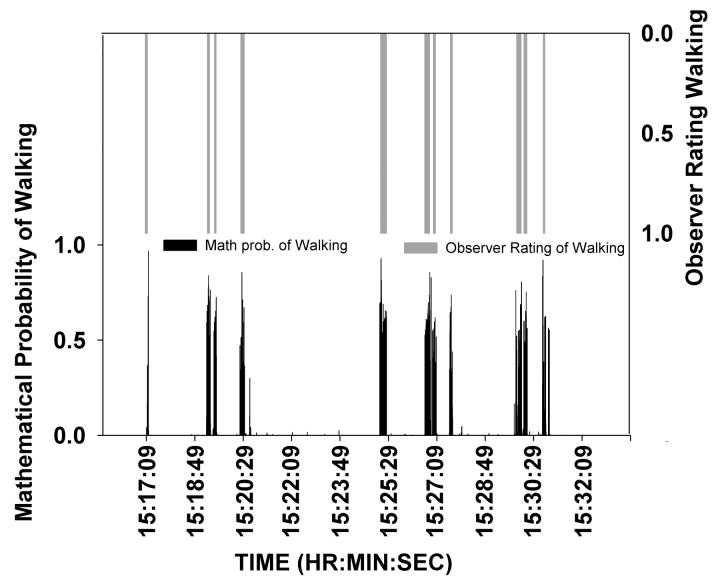
The continuous, high-frequency sampling of motor activity data also allows us to calculate dynamical entropy, h, which is comparable to the entropy measure mentioned above in the context of our animal studies. Dynamical entropy quantifies the predictability of a given level of activity based upon preceding patterns of activity. This acceleration-derived entropy measure captures, to our knowledge, a unique feature of human locomotor behavior, i.e. how sequences of acceleration events are organized in time. More importantly, we have already been able to use it to derive entropy “signatures” for specific and distinctive patterns of motor behavior. For example, in initial standardization studies, we generated average entropy values for motor behaviors that subjects exhibited in response to audio-taped instructions, e.g. walking, sitting, standing motionless, or exploring an environment. These entropy values were then used to generate mathematical probabilities that new subjects, uninstructed in the human BPM, were engaging in those motor behaviors. Figure 6 illustrates that videotape ratings of a subject's walking behavior corresponded precisely with the entropy-derived mathematical “signature” of walking. Given that dynamical entropy h can characterize disordered movement as well as perseverative movement as described above in our animal studies, it holds much promise as a potentially informative measure of how human motor behavior is organized across time.
Figure 6. Observed walking and entropy-derived probability.
The close correspondence between walking behavior as observed by video ratings (gray line) and entropy-derived probability of walking (black line) for one healthy human subject across a 15-minute session in the human BPM. The entropy-derived probability of walking corresponded exactly to the observed walking at each time-frame.
A measure of sequential organization that can be derived from the human BPM data and is completely analogous to our measurement of the organization of rodent locomotor data is the spatial scaling exponent, d, which, as in the case of the rodent work reviewed above, describes the geometric pattern of a subject's movement in the exploratory environment. Spatial dis derived in a near-identical manner to the rodent BPM (Paulus et al. 1990, Ralph et al. 2001). The series of x-y-coordinates derived from the digitized video images describes the spatial patterns of the subject's location and is used to calculate spatial d over specified time blocks.
Although the human BPM is in some respects a novel effort to develop a parallel to the animal open field paradigm, laboratory-based exploratory environments for humans have been reported previously. An early study examined the exploratory behavior of infants in a novel environment (Rheingold and Eckerman, 1969). Similarly, (Pierce and Courchesne 2001) quantified exploratory behavior in autistic children by rating videotapes of an eight-minute session where subjects were placed in a room with colorful and interactive objects. Ratings of decreased exploration were correlated with MRI-based measures of altered brain volumes in children with autism, suggesting that this exploratory paradigm was useful in detecting behavioral deficits that are associated with brain dysfunction.
Current Applications and Future Directions
The human BPM is one of the central measures we are using in an ongoing investigation of inhibitory deficits in bipolar mania. The original basis for developing the human BPM was to conduct parallel, cross-species studies of inhibitory problems that are features of the mania of BD and to extend the paradigm to other conditions such as schizophrenia, where multivariate assessment of motor behavior can reveal distinctive characteristics of the illness. An important aim of this study was to develop and validate rodent models of mania, which has been identified as a need in the literature (Einat 2006). This validation would be in part based upon the potential similarities between the motor activity of manic patients in the human BPM and the corresponding rodent BPM studies of mice that have been genetically or pharmacologically manipulated to create trait or state conditions of hyperdopaminergia. Thus, as noted above, the human BPM is an example of a “reverse-translational” approach to neuroscience research: whereas most paradigms that are eventually applied to both humans and animals are first developed in humans and then modified to be tested in animal models, the human BPM is unique in that it was developed as an analog to a widely used and highly influential animal paradigm, the open field as elaborated into the rodent BPM. Thus far it shows great promise in characterizing the motor behavior of human clinical populations, both in terms of some of the more straightforward measures such as accelerometry and video ratings, as well as the more complex measures of entropy and patterns of sequential movements in space.
We are currently administering the human BPM paradigm to manic BD patients and individuals with schizophrenia who have been hospitalized on an inpatient psychiatric unit for an acute exacerbation of their illness. In Figure 5 we illustrate representative case examples of human BPM data for our clinical populations as well as the non-patient cohort. The x-y coordinate tracings of the manic BD patient clearly show a very high level of activity in the BPM (Fig. 5b). Both the average acceleration and the number of transitions during the BPM session are substantially higher than those of the schizophrenia patient (Fig. 5c) or the healthy comparison subject (Fig. 5a). In addition, the manic patient exhibits markedly more interactions with the exploratory objects. The relatively low spatial d in the manic patient suggests that his motor behavior is characterized by long, straight movements from one area of the room to the next. This pattern of increased motor activity and increased exploratory behavior in combination with a reduced spatial d is comparable to what we have observed in DAT KD mice (see Fig 3) and mice administered GBR12909 (see Fig. 4), suggesting that DAT KD and GBR12909-treated mice may be intriguing candidates for genetic and pharmacological animal models of mania. In contrast, the schizophrenia patient exhibits very low motor activity, little exploration of objects, and a higher spatial d, signifying restricted and localized activity. The striking difference between the manic BD and the schizophrenia patient once again highlights the importance of multivariate assessment of activity, where measurement of multiple parameters may yield distinct “signatures” of locomotor activity that characterize and differentiate these two disorders. From a diagnostic perspective, the human BPM may be able to quantitatively assess an obvious and meaningful difference between two acutely ill populations who, during acute states, are often difficult to distinguish from one another because the behavioral presentation of both patient groups is dominated by psychotic and mood symptoms (Pini et al. 2004).
The potential objective, sensitive, and multivariate characterization of hyperactivity that is afforded by the human BPM offers many directions for future research. One obvious application would be to conduct pharmacological manipulations in parallel animal and human studies. For example, while the effects of stimulants on rodent motor behavior have been thoroughly characterized in the rodent BPM, studying stimulant-induced hyperactivity in the human BPM may help us further elucidate the behavioral features of an acute hyperdopaminergic state in healthy humans. Similarly, the human BPM may be useful in testing the efficacy of compounds for characterizing disorders that have hyperactivity as a central symptom. Such comparisons could include patients with BD, schizophrenia, schizoaffective disorder, and attention-deficit/hyperactivity disorder (ADHD) as well as developmental illnesses such as autism spectrum and impulse control disorders. As part of our current study on bipolar mania, we are re-testing manic subjects in the human BPM after several weeks of stabilization on psychotropic medications. We hypothesize that patients who are treated with a combination of an antipsychotic and mood stabilizer will show faster alleviation of symptoms of hyperactivity than those patients treated with a mood stabilizer alone, since the antipsychotic medications act directly as dopamine antagonists while mood stabilizers probably only indirectly modulate dopamine levels. While ours is a naturalistic study in which manic patients are not randomized to medications, an obvious future direction is to carry out randomized, controlled investigations of the effects of psychotropic compounds on hyperactivity, and to examine the time course of these effects. A possible limitation of the human BPM in longitudinal studies, however, is the potential effect of habituation, insofar as the human BPM ceases to be a totally novel environment with repeated exposures. The same problem is evident in longitudinal studies in rodents. One way to address this issue in a controlled fashion would be to design future studies where a group of subjects is first tested in a medicated state and re-tested after withdrawal from medications. These are obviously challenging studies to implement but they hold much promise for informing the field about the efficacy of psychotropic medications.
In conclusion, the human BPM is an important example of cross-fostering translational research. Given the importance of hyperactivity in many psychiatric disorders in general and in bipolar mania in particular, it is surprising that experimental approaches to measure locomotor behavior empirically in humans have not been more abundant in the literature. Our experience with locomotor behavior in rodents has shown that it is a complex phenotype that is not sufficiently characterized by quantifying only the amount of behavior. Instead, measures that quantify its temporal, spatial, and dynamic organization have proven to be valuable tools to differentiate the contributions of different neural transmitter systems on locomotor and exploratory behavior. Similarly, we predict that multivariate approaches to human locomotor and exploratory behavior will provide powerful insights into the neural bases of these behaviors and may provide new biomarkers as targets for the development of novel antimanic agents.
Acknowledgements
The authors thank Eliza Ferguson, Andrew Goey, Meegin Kincaid, Virginia Masten, Richard Sharp, and Rebecca Wershba for their invaluable contributions to this work. This work was supported by a grant from the National Institute of Mental Health (R01MH071916) and by the Veteran's Administration VISN 22 Mental Illness Research, Education, and Clinical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Adams LM, Geyer MA. LSD-induced alterations of locomotor patterns and exploration in rats. Psychopharmacology (Berl) 1982;77:179–85. doi: 10.1007/BF00431945. [DOI] [PubMed] [Google Scholar]
- Antoniou K, Kafetzopoulos E. A comparative study of the behavioral effects of d-amphetamine and apomorphine in the rat. Pharmacol Biochem Behav. 1991;39:61–70. doi: 10.1016/0091-3057(91)90398-l. [DOI] [PubMed] [Google Scholar]
- Barr AM, Lehmann-Masten V, Paulus M, Gainetdinov RR, Caron MG, Geyer MA. The selective serotonin-2A receptor antagonist M100907 reverses behavioral deficits in dopamine transporter knockout mice. Neuropsychopharmacology. 2004;29:221–8. doi: 10.1038/sj.npp.1300343. [DOI] [PubMed] [Google Scholar]
- Braff DL, Swerdlow NR, Geyer MA. Gating and habituation deficits in the schizophrenia disorders. Clin Neurosci. 1995;3:131–9. [PubMed] [Google Scholar]
- Bushnell PJ. Effects of scopolamine on locomotor activity and metabolic rate in mice. Pharmacol Biochem Behav. 1987;26:195–8. doi: 10.1016/0091-3057(87)90555-7. [DOI] [PubMed] [Google Scholar]
- Cabib S, Puglisi-Allegra S, Ventura R. The contribution of comparative studies in inbred strains of mice to the understanding of the hyperactive phenotype. Behav Brain Res. 2002;130:103–9. doi: 10.1016/s0166-4328(01)00422-3. [DOI] [PubMed] [Google Scholar]
- Collins C, Laird RI, Richards PT, Starmer GA, Weyrauch S. Aspirin-caffeine interaction in the rat. J Pharm Pharmacol. 1979;31:611–4. doi: 10.1111/j.2042-7158.1979.tb13602.x. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 1999;835:18–26. doi: 10.1016/s0006-8993(98)01258-x. [DOI] [PubMed] [Google Scholar]
- Crusio WE. Genetic dissection of mouse exploratory behaviour. Behav Brain Res. 2001;125:127–32. doi: 10.1016/s0166-4328(01)00280-7. [DOI] [PubMed] [Google Scholar]
- Diehl DJ, Gershon S. The role of dopamine in mood disorders. 1992;33:115–120. doi: 10.1016/0010-440x(92)90007-d. [DOI] [PubMed] [Google Scholar]
- Drai D, Golani I. SEE: a tool for the visualization and analysis of rodent exploratory behavior. Neurosci Biobehav Rev. 2001;25:409–26. doi: 10.1016/s0149-7634(01)00022-7. [DOI] [PubMed] [Google Scholar]
- Eilam D, Golani I. The ontogeny of exploratory behavior in the house rat (Rattus rattus): the mobility gradient. Dev Psychobiol. 1988;21:679–710. doi: 10.1002/dev.420210707. [DOI] [PubMed] [Google Scholar]
- Eilam D, Golani I. Home base behavior of rats (Rattus norvegicus) exploring a novel environment. Behav Brain Res. 1989;34:199–211. doi: 10.1016/s0166-4328(89)80102-0. [DOI] [PubMed] [Google Scholar]
- Eilam D, Golani I. Home base behavior in amphetamine-treated tame wild rats (Rattus norvegicus) Behav Brain Res. 1990;36:161–70. doi: 10.1016/0166-4328(90)90170-j. [DOI] [PubMed] [Google Scholar]
- Eilam D, Shefer G. The developmental order of bipedal locomotion in the jerboa (Jaculus orientalis): pivoting, creeping, quadrupedalism, and bipedalism. Dev Psychobiol. 1997;31:137–42. doi: 10.1002/(sici)1098-2302(199709)31:2<137::aid-dev6>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Einat H. Modelling facets of mania--new directions related to the notion of endophenotypes. J Psychopharmacol. 2006;20:714–22. doi: 10.1177/0269881106060241. [DOI] [PubMed] [Google Scholar]
- Emilien G, Maloteaux JM, Geurts M, Hoogenberg K, Cragg S. Dopamine receptors--physiological understanding to therapeutic intervention potential. 1999;84:133–156. doi: 10.1016/s0163-7258(99)00029-7. [DOI] [PubMed] [Google Scholar]
- File SE, Wardill AG. The reliability of the hole-board apparatus. Psychopharmacologia. 1975;44:47–51. doi: 10.1007/BF00421183. [DOI] [PubMed] [Google Scholar]
- Fink JS, Smith GP. Abnormal pattern of amphetamine locomotion after 6-OHDA lesion of anteromedial caudate. Pharmacol Biochem Behav. 1979;11:23–30. doi: 10.1016/0091-3057(79)90292-2. [DOI] [PubMed] [Google Scholar]
- Fitzgerald RE, Berres M, Schaeppi U. Validation of a photobeam system for assessment of motor activity in rats. Toxicology. 1988;49:433–9. doi: 10.1016/0300-483x(88)90029-7. [DOI] [PubMed] [Google Scholar]
- Flicker C, Geyer MA. Behavior during hippocampal microinfusions. IV. Transmitter interactions. Brain Res. 1982;257:137–47. doi: 10.1016/0165-0173(82)90009-1. [DOI] [PubMed] [Google Scholar]
- Franks RD, Adler LE, Waldo MC, Alpert J, Freedman R. Neurophysiological studies of sensory gating in mania: comparison with schizophrenia. Biol Psychiatry. 1983;18:989–1005. [PubMed] [Google Scholar]
- Friedman JN, Hurley RA, Taber KH. Bipolar disorder: imaging state versus trait. J Neuropsychiatry Clin Neurosci. 2006;18:296–301. doi: 10.1176/jnp.2006.18.3.296. [DOI] [PubMed] [Google Scholar]
- Geyer MA. Variational and probabalistic aspects of exploratory behavior in space: Four stimulant styles. Psychopharmacology Bulletin. 1982;18:48–51. [Google Scholar]
- Geyer MA. Approaches to the characterization of drug effects on locomotor activity in rodents. In: Adler MW, Cowan A, editors. Testing and Evaluation of Drugs of Abuse. Wiley-Liss, Inc; 1990. [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001;156:117–54. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Geyer MA, McIlwain KL, Paylor R. Mouse genetic models for prepulse inhibition: an early review. Mol Psychiatry. 2002;7:1039–53. doi: 10.1038/sj.mp.4001159. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Paulus M. Multivariate Analyses of Locomotor and Investigatory Behavior in Rodents. In: Ossenkopp K, Kavaliers M, Sanberg PR, editors. Mearusing Movement and Locomotion: From Invertebrates to Humans. Chapman & Hall; 1996. [Google Scholar]
- Geyer MA, Russo PV, Masten VL. Multivariate assessment of locomotor behavior: pharmacological and behavioral analyses. Pharmacol Biochem Behav. 1986;25:277–88. doi: 10.1016/0091-3057(86)90266-2. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Russo PV, Segal DS, Kuczenski R. Effects of apomorphine and amphetamine on patterns of locomotor and investigatory behavior in rats. Pharmacol Biochem Behav. 1987;28:393–9. doi: 10.1016/0091-3057(87)90460-6. [DOI] [PubMed] [Google Scholar]
- Gold LH, Koob GF, Geyer MA. Stimulant and hallucinogenic behavioral profiles of 3,4-methylenedioxymethamphetamine and N-ethyl-3,4-methylenedioxyamphetamine in rats. J Pharmacol Exp Ther. 1988;247:547–55. [PubMed] [Google Scholar]
- Gooding DC, Tallent KA. The association between antisaccade task and working memory task performance in schizophrenia and bipolar disorder. J Nerv Ment Dis. 2001;189:8–16. doi: 10.1097/00005053-200101000-00003. [DOI] [PubMed] [Google Scholar]
- Goodwin FK, Jamison KR. Manic-depressive illness. Oxford UP: 1990. [Google Scholar]
- Gordon D, Beck CH. Subacute apomorphine injections in rats: effects on components of behavioral stereotypy. Behav Neural Biol. 1984;41:200–8. doi: 10.1016/s0163-1047(84)90583-1. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Keith RA, Bhat RV. Differential sensitivity to lithium's reversal of amphetamine-induced open-field activity in two inbred strains of mice. Behav Brain Res. 2001;118:95–105. doi: 10.1016/s0166-4328(00)00318-1. [DOI] [PubMed] [Google Scholar]
- Grailhe R, Waeber C, Dulawa SC, Hornung JP, Zhuang X, Brunner D, Geyer MA, Hen R. Increased exploratory activity and altered response to LSD in mice lacking the 5-HT(5A) receptor. Neuron. 1999;22:581–91. doi: 10.1016/s0896-6273(00)80712-6. [DOI] [PubMed] [Google Scholar]
- Greenwood TA, Alexander M, Keck PE, McElroy S, Sadovnick AD, Remick RA, Kelsoe JR. Evidence for linkage disequilibrium between the dopamine transporter and bipolar disorder. Am J Med Genet. 2001;105:145–51. doi: 10.1002/1096-8628(2001)9999:9999<::aid-ajmg1161>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Hayden EP, Nurnberger JI., Jr. Molecular genetics of bipolar disorder. Genes Brain Behav. 2006;5:85–95. doi: 10.1111/j.1601-183X.2005.00138.x. [DOI] [PubMed] [Google Scholar]
- Kafkafi N, Lipkind D, Benjamini Y, Mayo CL, Elmer GI, Golani I. SEE locomotor behavior test discriminates C57BL/6J and DBA/2J mouse inbred strains across laboratories and protocol conditions. Behav Neurosci. 2003;117:464–77. doi: 10.1037/0735-7044.117.3.464. [DOI] [PubMed] [Google Scholar]
- Krebs-Thomson K, Geyer MA. Evidence for a functional interaction between 5-HT1A and 5-HT2 receptors in rats. Psychopharmacology (Berl) 1998;140:69–74. doi: 10.1007/s002130050740. [DOI] [PubMed] [Google Scholar]
- Krebs-Thomson K, Masten VL, Naiem S, Paulus MP, Geyer MA. Modulation of phencyclidine-induced changes in locomotor activity and patterns in rats by serotonin. Eur. J. Pharm. 1998;343:135–143. doi: 10.1016/s0014-2999(97)01557-4. [DOI] [PubMed] [Google Scholar]
- Kulkarni SK, Dandiya PC. Influence of chemical stimulation of central dopaminergic system on the open field behaviour of rats. Pharmakopsychiatr Neuropsychopharmakol. 1975;8:45–50. doi: 10.1055/s-0028-1094442. [DOI] [PubMed] [Google Scholar]
- Lat J. The spontaneous exploratory reactions as a tool for pharmacological studies: A contribution towards a theory of contracdictory results in psychopharmacology. In: Mikhelson M, LongoandZ V, editors. Pharmacology of Conditioning, Learning and Retention. Pergamon Press; Votava: 1965. [Google Scholar]
- Lehman-Masten V, Geyer MA. Spatial and temporal patterning distinguishes the locomotor activating effects of dizocilpine and phencyclidine in rats. Neuropharmacology. 1991;30:629–636. doi: 10.1016/0028-3908(91)90083-n. [DOI] [PubMed] [Google Scholar]
- Makanjuola RO, Hill G, Dow RC, Campbell G, Ashcroft GW. The effects of psychotropic drugs on exploratory and stereotyped behaviour of rats studied on a hole-board. Psychopharmacology (Berl) 1977;55:67–74. doi: 10.1007/BF00432819. [DOI] [PubMed] [Google Scholar]
- Marco EM, Llorente R, Perez-Alvarez L, Moreno E, Guaza C, Viveros The kappa-opioid receptor is involved in the stimulating effect of nicotine on adrenocortical activity but not in nicotine induced anxiety. Behav Brain Res. 2005;163:212–8. doi: 10.1016/j.bbr.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Stitzel JA, Romm E, Wehner JM, Collins AC. Nicotinic binding sites in rat and mouse brain: comparison of acetylcholine, nicotine, and alpha-bungarotoxin. Mol Pharmacol. 1986;30:427–36. [PubMed] [Google Scholar]
- McClung CA. Circadian genes, rhythms and the biology of mood disorders. Pharmacol Ther. 2007;114:222–32. doi: 10.1016/j.pharmthera.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meliska CJ, Loke WH. Caffeine and nicotine: differential effects on ambulation, rearing and wheelrunning. Pharmacol Biochem Behav. 1984;21:871–5. doi: 10.1016/s0091-3057(84)80067-2. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Sahakian BJ, Rubinsztein JS, Michael A, Rogers RD, Robbins TW, Paykel ES. Emotional bias and inhibitory control processes in mania and depression. Psychol Med. 1999;29:1307–21. doi: 10.1017/s0033291799001233. [DOI] [PubMed] [Google Scholar]
- Naylor E, Bergmann BM, Krauski K, Zee PC, Takahashi JS, Vitaterna MH, Turek FW. The circadian clock mutation alters sleep homeostasis in the mouse. J Neurosci. 2000;20:8138–43. doi: 10.1523/JNEUROSCI.20-21-08138.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenkopp K, Kavaliers M, Sanberg PR. Measuring Moevement and Locomotion: From Invertebrates to Humans. Chapman & Hall; 1996. [Google Scholar]
- Paulus MP, Dulawa SC, Ralph RJ, Geyer MA. Behavioral organization is independent of locomotor activity in 129 and C57 mouse strains. Brain Res. 1999;835:27–36. doi: 10.1016/s0006-8993(99)01137-3. [DOI] [PubMed] [Google Scholar]
- Paulus, Geyer MA. A scaling approach to find order parameters quantifying the effects of dopaminergic agents on unconditioned motor activity in rats. Prog Neuropsychopharmacol Biol Psychiatry. 1991;15:903–19. doi: 10.1016/0278-5846(91)90018-v. [DOI] [PubMed] [Google Scholar]
- Paulus, Geyer MA. The effects of MDMA and other methylenedioxy-substituted phenylalkylamines on the structure of rat locomotor activity. Neuropsychopharmacology. 1992;7:15–31. [PubMed] [Google Scholar]
- Paulus, Geyer MA. Quantitative assessment of the microstructure of rat behavior: I, f(d), the extension of the scaling hypothesis. Psychopharmacology (Berl) 1993a;113:177–86. doi: 10.1007/BF02245695. [DOI] [PubMed] [Google Scholar]
- Paulus, Geyer MA. Three independent factors characterize spontaneous rat motor activity. Behav Brain Res. 1993b;53:11–20. doi: 10.1016/s0166-4328(05)80262-1. [DOI] [PubMed] [Google Scholar]
- Paulus, Geyer MA. Assessing the Organization of Motor behavior: New Approaches Based on the Behavior of Complex Physical Systems. In: Ossenkopp K, Kavaliers M, Sanberg PR, editors. Measuring Movement and Locomotion: From Invertebrates to Humans. Chapman & Hall; 1996. [Google Scholar]
- Paulus MP, Geyer MA, Gold LH, Mandell AJ. Application of entropy measures derived from the ergodic theory of dynamical systems to rat locomotor behavior. Proc Natl Acad Sci U S A. 1990;87:723–7. doi: 10.1073/pnas.87.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Geyer MA, Mandell AJ. Statistical mechanics of a neurobiological dynamical system: The spectrum of local entropies (S(a)) applied to cocaine-perturbed behavior. Physica A. 1991;174:567–577. [Google Scholar]
- Pierce K, Courchesne E. Evidence for a cerebellar role in reduced exploration and stereotyped behavior in autism. Biological Psychiatry. 2001;49:655–664. doi: 10.1016/s0006-3223(00)01008-8. [DOI] [PubMed] [Google Scholar]
- Pini S, de Queiroz V, Dell'Osso L, Abelli M, Mastrocinque C, Saettoni M, Catena M, Cassano GB. Cross-sectional similarities and differences between schizophrenia, schizoaffective disorder and mania or mixed mania with mood-incongruent psychotic features. Eur Psychiatry. 2004;19:8–14. doi: 10.1016/j.eurpsy.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Post RM, Denicoff KD, Leverich GS, Altshuler LL, Frye MA, Suppes TM, Rush AJ, Keck PE, Jr., McElroy SL, Luckenbaugh DA, Pollio C, Kupka R, Nolen WA. Morbidity in 258 bipolar outpatients followed for 1 year with daily prospective ratings on the NIMH life chart method. J Clin Psychiatry. 2003;64:680–90. doi: 10.4088/jcp.v64n0610. quiz 738-9. [DOI] [PubMed] [Google Scholar]
- Powell SB, Lehmann-Masten VD, Paulus MP, Gainetdinov RR, Caron MG, Geyer MA. MDMA “ecstasy” alters hyperactive and perseverative behaviors in dopamine transporter knockout mice. Psychopharmacology (Berl) 2004;173:310–7. doi: 10.1007/s00213-003-1765-7. [DOI] [PubMed] [Google Scholar]
- Ralph-Williams RJ, Paulus MP, Zhuang X, Hen R, Geyer MA. Valproate attenuates hyperactive and perseverative behaviors in mutant mice with a dysregulated dopamine system. Biol Psychiatry. 2003;53:352–9. doi: 10.1016/s0006-3223(02)01489-0. [DOI] [PubMed] [Google Scholar]
- Ralph RJ, Paulus MP, Fumagalli F, Caron MG, Geyer MA. Prepulse inhibition deficits and perseverative motor patterns in dopamine transporter knock-out mice: differential effects of D1 and D2 receptor antagonists. J Neurosci. 2001;21:305–13. doi: 10.1523/JNEUROSCI.21-01-00305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph RJ, Paulus, Geyer MA. Strain-specific effects of amphetamine on prepulse inhibition and patterns of locomotor behavior in mice. J Pharmacol Exp Ther. 2001;298:148–55. [PubMed] [Google Scholar]
- Rao VS, Santos FA, Paula WG, Silva RM, Campos AR. Effects of acute and repeated dose administration of caffeine and pentoxifylline on diazepam-induced mouse behavior in the hole-board test. Psychopharmacology (Berl) 1999;144:61–6. doi: 10.1007/s002130050977. [DOI] [PubMed] [Google Scholar]
- Rebec GV, Bashore TR. Critical issues in assessing the behavioral effects of amphetamine. Neurosci Biobehav Rev. 1984;8:153–9. doi: 10.1016/0149-7634(84)90030-7. [DOI] [PubMed] [Google Scholar]
- Rheingold HL, Eckerman CO. The infant's free entry into a new environment. J. Exp. Child Psychol. 1969;8:271–83. doi: 10.1016/0022-0965(69)90102-7. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Masten VL, Caldwell S, Paulus MP, Low MJ, Geyer MA. Differential contributions of dopamine D1, D2, and D3 receptors to MDMA-induced effects on locomotor behavior patterns in mice. Neuropsychopharmacology. 2006;31:2349–58. doi: 10.1038/sj.npp.1301161. [DOI] [PubMed] [Google Scholar]
- Robbins TW. A critique of methods available for the measurement of spontaneous locomotor activity. In: Iversen LL, Iversen SD, editors. Handbook of Psychopharmacology. 1979. [Google Scholar]
- Sessions GR, Meyerhoff JL, Kant GJ, Koob GF. Effects of lesions of the ventral medial tegmentum on locomotor activity, biogenic amines and responses to amphetamine in rats. Pharmacol Biochem Behav. 1980;12:603–8. doi: 10.1016/0091-3057(80)90195-1. [DOI] [PubMed] [Google Scholar]
- Szechtman H, Ornstein K, Teitelbaum P, Golani I. The morphogenesis of stereotyped behavior induced by the dopamine receptor agonist apomorphine in the laboratory rat. Neuroscience. 1985;14:783–98. doi: 10.1016/0306-4522(85)90143-5. [DOI] [PubMed] [Google Scholar]
- Teicher MH. Actigraphy and motion analysis: new tools for psychiatry. Harvard Review of Psychiatry. 1995;3:18–35. doi: 10.3109/10673229509017161. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Lawrence JM, Barber NI, Finklestein SP, Lieberman H, Baldessarini RJ. Altered locomotor activity in neuropsychiatric patients. Progress in Neuropsychopharmacology and Biological Psychiatry. 1986;10:755–761. doi: 10.1016/0278-5846(86)90061-8. [DOI] [PubMed] [Google Scholar]
- Vawter MP, Freed WJ, Kleinman JE. Neuropathology of bipolar disorder. Biol Psychiatry. 2000;48:486–504. doi: 10.1016/s0006-3223(00)00978-1. [DOI] [PubMed] [Google Scholar]
- Vivometrics 2002. The LifeShirt System™.
- Walsh RN, Cummins RA. The open Field Test: A Critical Review. Psychological Bulletin. 1976;83:482–504. [PubMed] [Google Scholar]
- Wolff EA, Putnam FW, Post RM. Motor activity and affective illness. Arch Gen Psychiatry. 1985;42:288–294. doi: 10.1001/archpsyc.1985.01790260086010. [DOI] [PubMed] [Google Scholar]
- Young JW, Kerr LE, Kelly JS, Marston HM, Spratt C, Finlayson K, Sharkey J. The odour span task: A novel paradigm for assessing working memory in mice. Neuropharmacology. 2007;52:634–45. doi: 10.1016/j.neuropharm.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Oosting RS, Jones SR, Gainetdinov RR, Miller GW, Caron MG, Hen R. Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proc Natl Acad Sci U S A. 2001;98:1982–7. doi: 10.1073/pnas.98.4.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]