Abstract
A necessary condition for endothelialization of small diameter grafts is rapid and firm adhesion of endothelial cells upon exposure to flow. To retain integrins on the cell surface, we assessed the effects of trypsin concentration, the duration of trypsin incubation, and trypsin neutralization methods on endothelial cell adhesion. Human umbilical vein endothelial cells which were detached using 0.025% trypsin for five minutes and seeded onto glass pretreated with fibronectin had close to 100% cell retention when shear stresses as high as 200 dyn/cm2 were applied for two minutes. An equivalent level of cell retention was observed on fibronectin coated Teflon-AF™ for shear stresses up to 60 dyn/cm2 applied for 4 hours. Using 0.025% trypsin, initial cell spreading andα5β1 integrins are present on cells were increased relative to cells treated with 0.5% trypsin. After 1 hour of attachment, focal adhesions formed when low trypsin concentrations were used but were less evident with high trypsin concentrations. These results showed that low trypsin concentrations produced faster spreading spreading, a higher number of intact integrins, and rapid focal adhesion formation.
INTRODUCTION
There is considerable interest in promoting rapid and firm adhesion of endothelial cells (ECs) for a number of clinical applications, including seeding of vascular grafts and vascular tissue engineering [1]. ECs perform many vital functions: regulate platelet activation, adhesion, and aggregation; limit leukocyte adhesion; regulate smooth muscle cell migration and proliferation; and control blood flow and vessel tone [2-5].
EC adhesion to materials is mediated by adsorbed cell adhesion proteins, primarily fibronectin and/or vitronectin [6, 7]. Cell adhesion proteins bind to cell surface integrins, a family of heterodimeric transmembrane proteins consisting of α and β subunits. Integrins α5β1 and αVβ3 both play a major role in EC adhesion. Binding to extracellular matrix proteins activates integrins to associate with the actin cytoskeleton and eventually leads to integrin clustering into focal adhesions [8]. The formation of focal adhesions, which contain actin-associated proteins such as talin, vinculin, paxillin, and α-actinin, strengthens the adhesion of cells to the substrate [9].
Critical shear stress experiments are a common method used to quantify cell's adhesion strength to specific substrates. Prior to seeding a synthetic surface, ECs are usually treated with the proteolytic enzyme, trypsin, which cleaves proteins at the carboxyl side of the basic amino acids lysine and arginine, in order to remove cells from their culture substrate and hence be used for further applications [10]. Both α5β1 and αVβ3 integrins are sensitive to trypsin [11].
Of those studies that do report the trypsin concentrations and incubation times to detach cells, the trypsin concentrations to detach cells range from 0.05% to 0.5% [13-24] and incubation times range from 5 and 10 minutes [12-14] at either room (25°C) or physiological temperature (37°C). Taking the steps to neutralize the trypsin may also be critical for later cell attachment and spreading. The cell suspension is either centrifuged directly and resuspended in culture medium or a neutralizing solution containing serum or calcium ions is added to inhibit the trypsin before centrifugation.
While previous articles have reported relatively low critical shear stress values of 12−37 dyn/cm2 after 15 minutes to 1 hour of cell attachment [5, 6, 9, 14-17], no direct assessment is reported in the literature on the effect of trypsin on the critical shear stress needed to detach 50% of the cells from the surface.
In a clinical setting there is limited time for either cultured or freshly isolated ECs to strongly attach and spread on graft surfaces. Therefore, it is important to develop methods to promote rapid and strong cell adhesion. We have optimized in vitro conditions to yield very high adhesion strength after short periods of attachment to glass and Teflon-AF™. Differences in cell adhesion were characterized by the effect of trypsin on initial cell attachment, cell spreading, the number of intact integrins (α5β1 and αVβ3) present, and the localization of vinculin at 1 hour post attachment.
MATERIALS AND METHODS
Cell Culture
Human umbilical vein endothelial cells (HUVECs) (Cambrex BioScience Inc., Walkersville, MD) were grown to confluence in T-25 or T-75 polystyrene flasks (Becton Dickinson and Company, Franklin Lakes, NJ) with Endothelial Basal Media (EBM, Cambrex) supplemented with EGM SingleQuots (Cambrex) and 1X antibiotic/ antimycotics solution (Gibco, Carlsbad, CA). Cells were cultured in a tissue culture incubator with 95% air/5% CO2 at 37°C. HUVECs were used at passage 3−6 for all experiments.
After rinsing HUVECs with Dulbecco's Phosphate Buffered Saline solution (DPBS, Gibco) without Ca++ and Mg++, the conditions in Table 1 were examined to determine the effect of trypsin (Gibco) concentration, trypsin incubation time, and Trypsin Neutralizing Solution (TNS) (Cambrex). Once the cells were trypsinized and the appropriate neutralization solution was added, the cell suspension was immediately centrifuged, resuspended in either DPBS or serum free media (serum free media consisted of EBM and EGM SingleQuots minus the fetal bovine serum), and then used in specific experiments. For each of the trypsin concentrations, cell detachment was complete at the end of the incubation period.
Table 1.
Trypsin conditions used throughout paper.
| Condition # | Trypsin Concentration | Trypsin Incubation Time (minutes) | Neutralization Solution Used |
|---|---|---|---|
| 1 | 0.025% | 5 minutes at 37°C | TNS |
| 2 | 0.025% | 10 minutes at 37°C | TNS |
| 3 | 0.025% | 10 minutes at 37°C | DPBS |
| 4 | 0.5% | 5 minutes at 37°C | TNS |
| 5 | 0.5% | 10 minutes at 37°C | TNS |
| 6 | 0.5% | 10 minutes at 37°C | DPBS |
Substrates
Cell adhesion and spreading studies were performed using either standard 1” × 3” × 1 mm soda lime glass slides (Gold Seal, Portsmouth, NH) or Teflon-AF™ (DuPont, Wilmington, DE) films spun-cast onto standard glass microscope slides. Prior to use, the glass slides were cleaned by sonication with 2% PCC-54 detergent cleaning solution (Pierce, Rockford, IL) and a 1:1 mixture of MeOH:HCl as previously described and the Teflon-AF™ was spun-cast onto the clean microscope slides also as previously described [1]. All glass slides were pretreated with 1ml of DPBS containing both 10 μg/ml fibronectin (Sigma) and 200 μl of 2 mg/ml bovine serum albumin (BSA, Sigma) for 1 hour at 37°C, unless noted otherwise. Teflon-AF™ coated slides were incubated with 1 ml of 10 μg/ml fibronectin in DPBS for 1 hour at 37°C.
Cell Adhesion Strength and Cell Retention
Cell detachment conditions, summarized in Table 1, were used to determine the effect of trypsin conditions on initial adhesion and retention. ECs were resuspended in DPBS and attached to the surfaces for 5 minutes at room temperature. Images of adherent cells were obtained at 5 specific positions at 10X magnification using phase contrast microscopy (Nikon Diaphot, Tokyo, Japan). Next, slides were gently rinsed three times by slowly removing slides from DPBS at an angle. We had previously determined that three rinses were sufficient to remove non-specifically bound cells from the slides treated with BSA only. A second set of images was taken at the same five positions post dip rinse and the percent of adherent cells per field after rinsing was determined. Three experiments were performed for each of the conditions listed in Table 1.
After rinsing, the slide was placed in a variable height flow chamber which produces a range of shear stresses [12]. A set of 5 images was taken at 5 different channel heights along the chamber. Steady laminar flow was applied for two minutes through the use of a dual syringe pump. The total elapsed time from initial cell attachment to the onset of flow was typically 20 minutes. The flow media consisted of DPBS with 1.9% dextran (molecular weight, 2 × 106; Sigma) to produce a viscosity of 1.9 centipose, which exhibited Newtonian fluid behavior. The flow was set at a constant rate and the shear stress was computed by:
where μ is the media viscosity, w is the width of the flow channel, Q is the volumetric flow rate (ml/min), and H(x) is the height of the flow chamber as a function of position along the microscope slide [12]. Shear stresses typically ranged from 20 to 100 dyn/cm2. To produce shear stressed as high as 200 dyn/cm2, the flow rate was increased and viscosity was raised to 2.5 centipose (2.5% dextran), which still exhibited Newtonian fluid behavior. After flow exposure, images were taken at exactly the same positions as the pre-flow images, and the cells were counted in both images to determine the number of cells that remained adherent post-flow. Results were normalized to the number of cells present prior to rinsing. Experiments were performed in triplicate.
EC Adhesion to Glass and Teflon-AF™ Exposed to Four Hours of Flow
For four hour flow experiments, HUVECs were detached from flasks using low trypsin concentrations (0.025% trypsin for 5 minutes at 37°C and neutralizing with TNS), resuspended in serum-free media, and seeded onto glass or Teflon-AF™ slides for 1 hour at 37°C prior to flow. Before the 1 hour incubation time was over, the slides were assembled in a variable height flow chamber and images were taken at 5 positions at 5 different channel heights. The flow chamber was then connected to a circular flow loop [12] and flow was applied for 4 hours using EBM media with 2% fetal bovine serum (no additional growth factors were added to the media). After the 4-hour flow, images were taken again at the same exact locations as the pre-flow images. Cells were counted in the pre- and post-flow images in order to determine the percentage of cells that remained adherent post flow at the varying shear stress values. Both of the 4 hour flow experiments on glass and Teflon-AF™ were performed three times.
Cell Spreading
ECs were resuspended in serum free media after detachment using the conditions listed in Table 1 and seeded onto clean glass slides. One hour after seeding, the media was aspirated and replaced with media containing 2% fetal bovine serum (we found that the cells would not survive for more than a few hours without serum being present, data not shown). For each of the 6 conditions listed, images were taken using a phase contrast microscope at 20X magnification at various time points over a 24-hour period. The projected cell area and perimeter was measured using ImageJ (version 1.36, National Institutes of Health) software. For each condition, 5 images were obtained at each time point for each experiment. On average, 50−60 cells were examined for each time point for each experiment, and three experiments were performed for each of the 6 conditions listed in Table 1.
To assess the importance of protein synthesis [18], the cells were trypsinized using both the low and high trypsin concentrations, resuspended in serum free media containing 50 μg/ml of cycloheximide and allowed to incubate for 30 minutes with gentle rotation at room temperature. The cells were then seeded with 50 μg/ml cycloheximide added to the media and spreading was measured as described above.
Surface Expression of α5β1 and αVβ3 Integrins
The number of α5β1 and αVβ3 integrins present on ECs was measured after each of the trypsin conditions listed in Table 1. Detached cells were resuspended in DPBS, and incubated for 5 minutes. The cells were then incubated with 10 μg/ml of cycloheximide for 30 minutes in order to block protein synthesis [18]. The cells were then rinsed, incubated with 10% goat serum (blocking buffer, Sigma), incubated with either 10 μg/ml mouse-anti-α5β1 or 20 μg/ml mouse-anti-αVβ3 antibodies (Chemicon), rinsed, incubated with Alexa fluor 488 goat-anti-mouse secondary antibody (Invitrogen), rinsed, and postfixed in 10% formalin. The antibody concentration used was determined as the concentration to saturate the integrin binding sites (data not shown).
Quantum Simply Cellular site density calibration standards (Bangs Laboratory, Inc., Fishers, IN) were used to make a calibration curve for the flow cytometry results. The standards consisted of 5 sets of latex beads with increasing numbers of antibody binding sites. The beads were incubated with the primary and secondary antibodies under conditions identical to those used with the cells.
Fluorescence intensity per bead produced by the bound antibody was measured using a FACStar Plus flow cytometer (Becton Dickinson, San Jose, CA). Data were typically collected for 10,000 cells or beads for each condition. In addition, an isotype control was used for each sample condition, and the mean fluorescent intensity found for the isotype control was subtracted from the mean fluorescent intensity of the antibody bound cells in order to compensate for background fluorescence.
A calibration curve was constructed based on the fluorescent intensities of the 5 calibration beads. For each experiment, the calibration curves were linear from background to 1.7 million antibody binding sites per bead, and r2 ranged from 0.93 to 0.99. Three experiments were done to determine the number of αVβ3 integrins and five experiments were done to determine the number of α5β1 integrins present.
Vinculin Immunofluorescence
To detect vinculin localization, cells were allowed to attach for one hour and were fixed for 10 minutes with 3.7% formaldehyde and permeabilized with 0.2% Triton X-100 (Sigma) for 5 minutes. The cells were rinsed with DPBS and incubated for 30 min at 37°C with blocking buffer (10% goat serum) to prevent non-specific binding. The primary antibody, mouse anti-human vinculin antibody (Sigma), was incubated with the cells for 1 hour at 37°C in 10% goat serum. This was followed by incubation for 45 minutes with Alexa Fluor 488 secondary antibody. The slides were mounted with coverslips and images were captured with a confocal laser scanning microscope (LSM 510, Carl Zeiss Inc., Thornwood, NY). ImageJ was used to determine the number of focal adhesions and size of focal adhesions present from the imaged cells.
Statistical Analysis
Statview 5.0.1 was used to compare data to determine the statistical differences between trypsin conditions. T-tests were performed to compare the effect of trypsin concentration on spreading when cycloheximide was present and to compare the number and size of focal adhesions present. One and two-way ANOVA plus Fisher protected least square difference (PLSD) post hoc analysis was conducted to determine significance levels for all other experiments. The Fisher PLSD compares the means of more than 2 groups with comparisons of each possible pair. All statistics are reported as the mean ± the standard error.
Results
Effect of Trypsin Conditions on Cell Adhesion and Retention
The effect of trypsin conditions on initial cell adhesion was examined by dip-rinsing glass slides three times in DPBS to remove non-specifically adherent cells after 5 minutes of cell attachment. Preliminary experiments indicated that three rinses would completely remove cells resting on a surface coated with BSA. Trypsin concentration had the largest effect (p<0.01) on initial cell attachment (Figure 1). At the low trypsin concentration of 0.025%, time and neutralization solution (TNS versus DPBS) did not affect cell attachment. When the trypsin concentration was increased to 0.5%, the lack of TNS reduced cell attachment (p<0.01).
Figure 1. Effect of trypsin conditions on the percentage of cells adherent at 5 minutes post cell seeding.
Cells were detached with the various conditions listed in Table 1 and attached onto fibronectin/BSA coated glass slides for 5 minutes. Cells underwent three dip rinses. Specific locations were analyzed before and after rinsing and the percentage of cells that remained was determined. There was a statistically significant effect of trypsin concentration on attachment (* p < 0.01), and a statistically significant effect of neutralizing solution used at the high trypsin concentration on attachment (** p < 0.01).
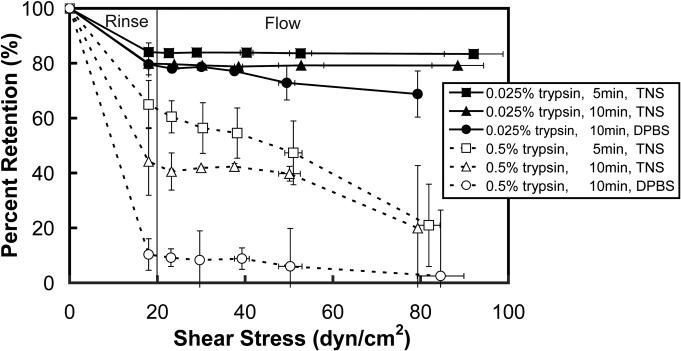
Figure 2 shows the combined effect of rinsing (denoted as Rinse) and exposure to laminar flow (denoted as Flow) observed under different trypsin conditions. The shear stress of 18 dyn/cm2 during rinsing was estimated for a falling film of 1 ml [19]. Our results indicate that cell retention after flow is dependent on the concentration of trypsin used to detach the cells. In addition, we found that when cells were trypsinized using the low trypsin concentrations, cell detachment was under 5% at 200 dyn/cm2 (data not shown).
Figure 2. Effect of trypsin conditions on the strength of cell adhesion.
Cells were detached using the 6 different trypsin conditions and allowed to adhere to a fibronectin/BSA coated glass slide for 20 minutes before rinsing and exposure to laminar flow. Images were taken of the cells at specific locations before and after the rinse and flow in order to determine the percent of cell retention (y-axis). The point to the left of the line is the cell detachment due to the rinse, and the points to the right of the line are cell detachment due to flow.
Cell Spreading
Cell area was measured for the 6 trypsin conditions at 6 different time points (Figure 3A). Cell area was very similar for all 6 conditions at time equal to 10 minutes, but for attachment times between 30 minutes and 3 hours a distinguishable difference can be seen for the cells that were trypsinized using the two different concentrations. Spreading is much greater using the mild trypsin concentration. By 24 hours post cell seeding (1440 minutes), the cell area was independent of the detachment conditions. The duration of trypsinization and the neutralization method did not affect spreading.
Figure 3A. Cell spread area versus time after attachment.
All conditions were performed on fibronectin/BSA coated glass slides. Cells were detached using the 6 different trypsin conditions listed in Table 1. After attachment, images were taken at 6 different time points and the projected area per cell determined. The results indicate that trypsin concentration had the largest effect on subsequent cell spreading.
In order to better understand the shape of the cells as they spread we measured the perimeter/area, over time for the 6 conditions (Figure 3B). Increasing values of perimeter/area are consistent with pseudopod extension occurred because the perimeter increased faster than the area. When the harsher trypsin conditions were used there appeared to be an increase in pseudopod extension between 90 minutes and 3 hours. After this time period the cell body subsequently filled in the area between the extensions as can be seen by the smaller perimeter/area ratios. Relative to cells that were detached with 0.025% trypsin, cells that were detached with 0.5% trypsin spread more slowly during the first 3 hours after reattachment. After 24 hours of attachment, the cell areas were the same for cells detached using both treatments.
Figure 3B. Cell perimeter/area over time for the 6 different trypsin conditions.
The individual cell areas found in Figure 3A were divided by their cell perimeters in order to determine the ratios for the different trypsin conditions over time. The results indicate that the harsher trypsin conditions resulted in larger ratios.
In order to assess whether expression of new integrins occurred during spreading, cell spreading was measured for cells incubated with and without cycloheximide, which inhibits protein synthesis [18]. During the first three hours of attachment, cyclocheximide treatment did not affect cell spreading (Table 2). By 24 hours after attachment, the projected cell area was reduced in the presence of cycloheximide only for cells detached using the high trypsin concentrations. Thus for cells treated under high trypsin concentrations, the increase in cell area between 3 and 24 hours is due, in part, to the synthesis of new proteins. In addition, we found that the perimeter/area values increased at 24 hours for cells incubated with cycloheximide relative to the values when cycloheximide was absent.
Table 2.
Percent increase in cell area 1, 3 and 24 hours after plating with or without cycloheximide from cells trypsinized with either 0.025% or 0.5% trypsin. (Values are mean ± SEM for n = 3; * p < 0.01 relative to control, cells not treated with cycloheximide and treated under the same conditions.)
| Cell Area Relative to Cell Area without Cylcoheximide Treatment | ||
|---|---|---|
| Time, hours | 0.025% | 0.5% |
| 1 | 1.00 ± 0.03 | 0.83 ± 0.06 |
| 3 | 1.06 ± 0.08 | 0.96 ± 0.03 |
| 24 | 0.77 ± 0.10 | 0.66 ± 0.02* |
Effect of Trypsin Conditions on the Level of α5β1 and αVβ3 Integrins
ECs adhere to adsorbed fibronectin by integrin complexes α5β1 and αVβ3 [6, 20]. A set of calibration antibody binding beads were used with flow cytometry to quantify the number of intact α5β1 and αVβ3 integrins on the cells after the various trypsin treatments listed in Table 1. The integrin expression level declined significantly above passage 4 (data not shown), which is consistent with previous reports [21]. To correct for the effect of passage number and inter-experimental variation in receptor number, the flow cytometry results in Figures 4A and 4B were normalized by the average number of receptors per cell within each experiment.
Figure 4. Number of cell surface α5β1 (A) and αVβ3 (B) integrins.
Cells were detached using the 6 different trypsin conditions listed in Table 1. The number of cell surface integrins were quantified using calibration beads and flow cytometry as described in Methods. There was a statistically significant effect (* p<0.001 ) of trypsin concentration on number of α5β1 integrins but no effect of trypsin on αVβ3 integrins. No other condition showed an effect.
The trypsin concentration significantly altered α5β1 expression (p<0.001) (Figure 4A). In contrast, the incubation time and neutralization solution had no significant effect on the expression of α5β1. Although reports indicate that αVβ3 is sensitive to trypsin [14], the number of αVβ3 integrins was not affected by the various trypsin conditions (Figure 4B).
Vinculin Localization
To evaluate whether the trypsin conditions affected focal adhesion formation, the cells detached using the different conditions were seeded onto fibronectin coated glass slides for 1 hour and immuno-stained for vinculin. ECs that were detached with 0.025% trypsin formed classical focal adhesions, evident by the presence of vinculin after 1 hour of attachment, but cells trypsinized using higher trypsin concentrations did not form as many focal adhesions at this time point, as can be seen in Figures 5A and B. To support these qualitative results, we quantified the number, size, and area occupied by focal adhesions (Table 3). Treatment of cells with the high trypsin concentrations reduced the percentage of cells that contained focal adhesions, the number of focal adhesions per cell and the average size of the focal adhesions (Table 3).
Figure 5. A. Vinculin staining for a cell trypsinized using 0.025% trypsin. B. Vinculin staining for cells detached with 0.5% trypsin.
Vinculin was detected by immunofluorescence in cells attached for 1 hour. All conditions were performed on fibronectin/BSA coated glass slides. Focal contacts are visible in Figure A, but are less evident in Figure B.
Table 3.
A quantitative analysis of the average percent of cells with focal adhesions, average number of focal adhesion, and average size of focal adhesions evident by vinculin immunostaining for cells trypsinized with either 0.025% or 0.5% trypsin. (Values are mean ± SEM for n=3; * p < 0.05 for a one sided t-test compared to results for cells detached with 0.025% trypsin.)
| Trypsin Concentration | Average % of cells with focal adhesions | Average # of focal adhesions per cm2 | Average size of focal adhesions (μm2) |
|---|---|---|---|
| 0.025% | 100 ± 0 | 312.2 ± 88.4 | 0.91 ± 0.06 |
| 0.5% | 37.8 ± 23.2 | 55.1 ± 9.6* | 0.55 ± 0.06* |
EC Adhesion to Glass and Teflon-AF™ Exposed to Four Hours of Flow
The effect of the mild trypsin detachment conditions on cell adhesion to glass and Teflon-AF™ was tested after the cells attached for 1 hour and were exposed to a range of shear stresses for 4 hours. The percentage of cells that remained adherent was similar on both surfaces for shear stresses below 60 dyn/cm2 (Figure 6). Exposure to shear stresses between 60 and 90 dyn/cm2 caused a cell loss of 10% from glass. In contrast, EC detachment from Teflon-AF™ at 60 dyn/cm2 was 40%. Nevertheless, the percentage of cells adherent to Teflon-AF™ is much greater than reported previously for much shorter term flow [5, 6, 9, 14-17].
Figure 6. Cells attached to glass or Teflon-AF™ for 1 hour and then exposed to a range of shear stress values for 4 hours.
Cells detached with 0.025% trypsin for 5 minutes and neutralized with TNS were allowed to adhere to both glass slides coated with fibronectin/BSA mixture, and Teflon-AF™ coated with fibronectin.
Discussion
The current study shows that high trypsin concentrations significantly reduced the cell's ability to form adhesive bonds with adsorbed cell adhesion proteins by decreasing the number of functional integrins available on the cell membrane. Preventing integrin damage with low trypsin concentrations resulted in substantially improved cell adhesion. Therefore, decreasing integrin damage or loss represents a promising strategy for promoting firm EC adhesion.
Only one prior study quantified the role of trypsin treatment on the number of cell surface receptors that are responsible for adhesion [11]. This study found that when 2.5 mg/ml (0.25%) of trypsin is incubated with HUVECs for 20 minutes, 90% of the fibronectin-specific receptors are cleaved [11]. Proteolysis of VCAM-1 (vascular cell adhesion molecule-1) and E-selectin occurs due to trypsin, and the degradation of VCAM-1 can be diminished when the trypsin concentrations decreased from 0.5% to 0.01% [22]. However, no one has systematically examined the trypsin conditions needed to effectively detach ECs and optimize adhesion strength.
In the current study we found that adhesion strength was improved significantly by trypsinization conditions much lower than those that are generally reported; i.e. cells detached completely from plastic surfaces following incubation with 0.025% trypsin for 5 minutes. This low trypsin concentration can retain close to 100% of ECs exposed to 200 dyn/cm2 of shear stress after only 20 minutes of attachment. This level of cell retention at such high shear stress has only been reported for NIH3T3 cells that were adherent for 16 hours and chondrocytes that were adherent for 80 minutes [8, 21].
Cells can be detached without trypsin, using 1mM EGTA (Ethyleneglycol-bis(β-aminoethyl)-N,N,N',N'-tetraacetic Acid) in a Ca++ and Mg++ free solution for 15 minutes [14]. This method caused an increase in the critical shear stress from 37 to 59 dyn/cm2 when BAECs were adherent for 15 minutes to glass with preadsorbed fibronectin [14]. This method for detachment slightly enhanced cell adhesion, but did not enhance it to the degree that we report here. However, EGTA does not always result in complete cell detachment within the 15 minutes and sometimes the cells detach as clumps. This incomplete detachment may explain the lower critical shear stress values compared to the values that we are currently reporting; the EGTA alone may detach a subpopulation of weakly adherent cells and hence when they are seeded they cannot adhere at high shear stresses. On the other hand, the current detachment method is highly reproducible since all cells detached and adhesion strength was much higher than physiological levels.
Adhesion strength is dependent upon the initial integrin-ligand binding and is subsequently strengthened by conformational changes to the integrin-ECM complex, clustering of receptors, and focal contact formation [8]. Mild trypsin concentrations used to detach cells resulted in more cell surface integrins specific for fibronectin binding, which facilitated rapid spreading of the cells and formation focal contacts. These results are consistent with rapid and firm adhesion assessed in the flow experiments.
The results from the initial cell attachment experiment (Figure 1) suggest that adhesion is sensitive to both the concentration of trypsin and the neutralizing solution used to treat cells after trypsinization. However, the number of α5β1 integrins present was affected only by the concentration of trypsin used and not by the neutralizing method (Figure 4A). It is plausible that trypsin can both cleave integrins and alter the α5β1 site on the cell surface without removing the α5β1 site. The high trypsin concentrations cleaved many more α5β1 integrins compared to the lower trypsin concentrations. The cells trypsinized with the high concentration and not neutralized may have many cleaved integrins and damaged integrins, while the cells trypsinized with harsh trypsin and neutralized may have cleaved integrins but less damaged ones. This may explain why there was no apparent difference in the number of integrins present post-trypsinization with high trypsin concentrations and neutralization solution, but there is a difference in the attachment of the cells. For example, the cells may have the same number of integrins present (cells trypsinized with the same concentration) but some of those integrins may be damaged from the lack of neutralization and hence will prevent cells from adhering. This could be assessed using antibodies with different epitopes for the specific integrins.
The above claim of integrins present is supported by flow cytometry data (Figure 4) as well as the spreading data presented in Figures 3A, 3B, and Table 2. The cells that were trypsinized using mild trypsin conditions spread much faster than the harshly trypsinized cells. However, after 24 hours of attachment, cells detached using high trypsin concentrations reached projected areas comparable to areas of cells detached using the low trypsin concentrations. These results suggest that the smaller number of integrins on cells detached with low trypsin concentrations initially produced slower spreading, but at later times, new receptor synthesis enhanced spreading. Table 2 supports this claim because the cells that were treated with cycloheximide did not show an increase in projected area between 3 and 24 hours compared to the cells that were not treated with cycloheximide. Cycloheximide is known to block protein synthesis [18] and if the cells are not synthesizing new integrins then the amount that they spread is due to the number of integrins, and other cell proteins, present after trypsinization.
In addition, Figure 3B shows how the perimeter/area changes with time for the 6 trypsin conditions used. The higher trypsin concentrations produced a greater increase in the perimeter/area ratio at 3 hours. These data are consistent with data presented by Reinhart-King et al. in which they also found that when the ligand density decreased they had an increase in perimeter/area [23]. In our cases, this can be explained by the reduced number of integrins present after the cells were treated with 0.5% trpysin. Over time when more integrins are expressed the cells increased their projected area and the hence the perimeter/area ratio decreased.
Focal adhesion proteins, such as vinculin, connect the ECM with the actin cytoskeleton by interactions with the cytoplasmic portion of integrins. Focal adhesion formation occurs very quickly (less than 30 minutes) after integrin ligation due to trypsin [9] . We also found this to be true for cells that were trypsinized using mild conditions; however, a very small amount of focal adhesion formation was evident for the cells that were harshly trypsinized. Further, since vinculin contributes about 30% of the cell's adhesion strength [8] vinculin recruitment with low trypsin concentrations may contribute to strong adhesion.
Commonly employed vascular graft materials have substantially different surface characteristics than a glass slide. The 4-hour flow experiment on Teflon-AF™ (Figure 7) is a good demonstration of the high levels of cell retention that can be obtained on low surface energy surfaces that mimic the synthetic vascular graft is ePTFE [1]. We obtained more than 60% adherence when shear stresses reached 100 dyn/cm2 compared to previous studies that had close to identical parameters which found only 50% adherence at 17.7 dyn/cm2 [1]. For this longer term flow experiment we allowed the cells to adhere for 1 hour before exposing to flow, which allowed the cells time to spread out and firmly adhere, but is still a reasonable time frame for adherence. In addition, it should be noted that even longer term studies would need to be done in order to be sure that endothelial cells will remain adherent to Teflon-AF™, however, this initial study was done in order to see if the data we obtained using mild trypsin conditions with glass surfaces could be translatable to surfaces similar to ones used for synthetic vascular grafts. These data are helpful for future studies to translate these conditions to mimic in vivo situations. We are currently extending the mild trypsin technique to cell adhesion studies on small diameter ePTFE vascular grafts.
Clinically, cells may be cultured or harvested from several sources, including a patient's adipose tissue, and immediately used to seed a vascular graft. In this situation, the cells will likely be exposed to higher concentrations of proteolytic enzymes for longer times than our low trypsin treatments which may affect adhesion; thus, some other form of adhesion enhancement may be necessary. If high trypsin concentrations or other harsh enzymatic digestion or detachment methods are used, it may be necessary to supplement integrin-mediated adhesion to permit firm and rapid attachment. Preliminary experiments suggest that adhesion can be enhanced by means of the dual ligand approach in which the cells are incubated with RGD-streptavidin and the synthetic surface coated with a combination of fibronectin and biotinylated bovine serum albumin [1, 24].
CONCLUSIONS
The results of the study suggest that more integrins remain intact than when cells are detached from substrates by 5 minute incubations with trypsin concentrations of 0.025%. As a result, cells form rapid and firm adhesion to glass and Teflon-AF™ coated glass surfaces, with adhesion strengths beyond the level of forces produced physiologically. This enhancement in adhesion takes us one step closer to making synthetic vascular grafts lined with firmly adherent ECs.
ACKNOWLEGEMENT
The authors would like to thank Dr. Theodore Slotkin for his assistance with the statistical analysis. The financial support of NIH grant HL-44972 and an NIH Biotechnology training grant (GM8555) fellowship to M.A.B. is also greatly appreciated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Anamelechi CC, Truskey GA, Reichert WM. Mylar and Teflon-AF as cell culture substrates for studying endothelial cell adhesion. Biomaterials. 2005;26:6887–96. doi: 10.1016/j.biomaterials.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 2.Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS, Barnathan ES, McCrae KR, Hug BA, Schmidt AM, Stern DM. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- 3.Jaffe EA. Cell Biology of Endothelial-Cells. Human Pathology. 1987;18:234–239. doi: 10.1016/s0046-8177(87)80005-9. [DOI] [PubMed] [Google Scholar]
- 4.Seifalian AM, Tiwari A, Hamilton G, Salacinski HJ. Improving the clinical patency of prosthetic vascular and coronary bypass grafts: The role of seeding and tissue engineering. Artificial Organs. 2002;26:307–320. doi: 10.1046/j.1525-1594.2002.06841.x. [DOI] [PubMed] [Google Scholar]
- 5.Sumpio BE, Riley JT, Dardik A. Cells in focus: endothelial cell. International Journal of Biochemistry & Cell Biology. 2002;34:1508–1512. doi: 10.1016/s1357-2725(02)00075-4. [DOI] [PubMed] [Google Scholar]
- 6.Ellis PD, Metcalfe JC, Hyvonen M, Kemp PR. Adhesion of endothelial cells to NOV is mediated by the integrins alpha v beta 3 and alpha 5 beta 1. Journal of Vascular Research. 2003;40:234–243. doi: 10.1159/000071887. [DOI] [PubMed] [Google Scholar]
- 7.Mathur AB, Chan BP, Truskey GA, Reichert WM. High-affinity augmentation of endothelial cell attachment: long-term effects on focal contact and actin filament formation. J Biomed Mater Res A. 2003;66:729–37. doi: 10.1002/jbm.a.10581. [DOI] [PubMed] [Google Scholar]
- 8.Gallant ND, Michael KE, Garcia AJ. Cell adhesion strengthening: Contributions of adhesive area, integrin binding, and focal adhesion assembly. Molecular Biology of the Cell. 2005;16:4329–4340. doi: 10.1091/mbc.E05-02-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plopper G, Ingber DE. Rapid induction and isolation of focal adhesion complexes. Biochem Biophys Res Commun. 1993;193:571–8. doi: 10.1006/bbrc.1993.1662. [DOI] [PubMed] [Google Scholar]
- 10.Olsen JV, Ong SE, Mann M. Trypsin cleaves exclusively C-terminal to arginine and lysine residues. Mol Cell Proteomics. 2004;3:608–14. doi: 10.1074/mcp.T400003-MCP200. [DOI] [PubMed] [Google Scholar]
- 11.Akiyama SK, Yamada KM. The interaction of plasma fibronectin with fibroblastic cells in suspension. J Biol Chem. 1985;260:4492–500. [PubMed] [Google Scholar]
- 12.Truskey GA, Proulx TL. Relationship between 3T3 Cell Spreading and the Strength of Adhesion on Glass and Silane Surfaces. Biomaterials. 1993;14:243–254. doi: 10.1016/0142-9612(93)90114-h. [DOI] [PubMed] [Google Scholar]
- 13.Iuliano DJ, Saavedra SS, Truskey GA. Effect of the Conformation and Orientation of Adsorbed Fibronectin on Endothelial-Cell Spreading and the Strength of Adhesion. Journal of Biomedical Materials Research. 1993;27:1103–1113. doi: 10.1002/jbm.820270816. [DOI] [PubMed] [Google Scholar]
- 14.Xiao Y, Truskey GA. Effect of receptor-ligand affinity on the strength of endothelial cell adhesion. Biophysical Journal. 1996;71:2869–2884. doi: 10.1016/S0006-3495(96)79484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feugier P, Black RA, Hunt JA, How TV. Attachment, morphology and adherence of human endothelial cells to vascular prosthesis materials under the action of shear stress. Biomaterials. 2005;26:1457–1466. doi: 10.1016/j.biomaterials.2004.04.050. [DOI] [PubMed] [Google Scholar]
- 16.Chan BP, Reichert WM, Truskey GA. Effect of streptavidinbiotin on endothelial vasoregulation and leukocyte adhesion. Biomaterials. 2004;25:3951–3961. doi: 10.1016/j.biomaterials.2003.10.077. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez P, Daculsi R, Remy-Zolghadri M, Bareille R, Bordenave L. Endothelial cells cultured on engineered vascular grafts are able to transduce shear stress. Tissue Engineering. 2006;12:1–7. doi: 10.1089/ten.2006.12.1. [DOI] [PubMed] [Google Scholar]
- 18.Lusska A, Wu L, Whitlock JP., Jr. Superinduction of CYP1A1 transcription by cycloheximide. Role of the DNA binding site for the liganded Ah receptor. J Biol Chem. 1992;267:15146–51. [PubMed] [Google Scholar]
- 19.Bird S, Lightfoot Transport Phenomena. 2002:45. [Google Scholar]
- 20.Juliano D, Wang YQ, Marcinkiewicz C, Rosenthal LA, Stewart GJ, Niewiarowski S. Disintegrin interaction with alpha(v)beta(3) integrin on human umbilical vein endothelial cells: Expression of ligand-induced binding site on beta(3) subunit. Experimental Cell Research. 1996;225:132–142. doi: 10.1006/excr.1996.0164. [DOI] [PubMed] [Google Scholar]
- 21.Kurtis MS, Schmidt TA, Bugbee WD, Loeser RF, Sah RL. Integrin-mediated adhesion of human articular chondrocytes to cartilage. Arthritis Rheum. 2003;48:110–8. doi: 10.1002/art.10704. [DOI] [PubMed] [Google Scholar]
- 22.Grabner R, Till U, Heller R. Flow cytometric determination of E-selectin, vascular cell adhesion molecule-1, and intercellular cell adhesion molecule-1 in formaldehyde-fixed endothelial cell monolayers. Cytometry. 2000;40:238–44. doi: 10.1002/1097-0320(20000701)40:3<238::aid-cyto9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Reinhart-King CA, Dembo M, Hammer DA. The dynamics and mechanics of endothelial cell spreading. Biophys J. 2005;89:676–89. doi: 10.1529/biophysj.104.054320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan BP, Chilkoti A, Reichert WM, Truskey GA. Effect of streptavidin affinity mutants on the integrin-independent adhesion of biotinylated endothelial cells. Biomaterials. 2003;24:559–70. doi: 10.1016/s0142-9612(02)00367-8. [DOI] [PubMed] [Google Scholar]