Abstract
Interleukin-6 (IL-6) is a major survival factor for malignant plasma cells. In patients with multiple myeloma (MM), cell lines whose survival and proliferation are dependent on addition of exogenous IL-6 have been obtained. We show that tumor necrosis factor α (TNF-α) is also a survival factor for myeloma cell lines, although less potent than IL-6. The survival activity of TNF-α is not affected by anti-IL-6 or anti-gp130 monoclonal antibodies (mAbs). TNF-α also induces myeloma cells into the cell cycle and promotes the long-term growth of malignant plasma cell lines. As TNF-α is produced in patients with MM and associated with a poor prognosis, these results suggest that anti-TNF-α therapies could be useful in this disease.
Keywords: Antibodies, Monoclonal; pharmacology; Apoptosis; drug effects; immunology; Cell Division; drug effects; Cell Survival; drug effects; Humans; Interleukin-6; biosynthesis; immunology; pharmacology; Multiple Myeloma; Recombinant Proteins; pharmacology; Tumor Cells, Cultured; Tumor Necrosis Factor-alpha; pharmacology
INTRODUCTION
IL-6 and more generally the gp130 IL-6 transducer-activating cytokines have been shown to be the major survival and proliferation factors for malignant human plasma cells (1–5). In particular, cell lines whose survival and proliferation are dependent on the addition of IL-6 can be reproducibly obtained from patients with multiple myeloma (6–8).
In an attempt to identify further tumoral survival and growth factors in this disease, we studied the effect of TNF-α. Indeed, preliminary reports indicated that this cytokine might promote the proliferation of some myeloma cell lines (7–9). It is important to clarify the role of TNF-α in multiple myeloma because this cytokine may have either an apoptotic or a survival activity. The mechanisms leading to these two opposite activities are being better and better understood. The TNF-dependent trimerization of TNF receptors (TNF-R) may lead to the recruitment of adapter proteins TRADD (TNF-R1 associated death domain protein) and FADD (Fas-associated death domain protein) or RIP (receptor interacting protein) resulting in activation of the caspase cascade (10,11) and further to apoptosis. On the opposite, the recruitment of TRADD, RIP and TRAF2 (TNFR associated factor 2) leads to the activation of NF-kappa B (12) which has been shown to play an essential role in preventing apoptosis (13–15).
We report here that TNF-α is not an apoptotic factor but a survival and proliferation factor of cytokine-dependent myeloma cell lines by a mechanism that is not related to the gp130-IL-6 transducer. These results suggest that this cytokine might be involved in the survival and proliferation of myeloma cells in vivo.
MATERIALS AND METHODS
Cytokines and antibodies
Purified Escherichia coli-expressed human rIL-6 was kindly provided by Dr Vita and Dr Ferrara (Sanofi, Elf biorecherches, Labège, France). Human purified rTNF-α and neutralizing anti-TNF-α mAb (mouse IgG3) were purchased from Boehringer Mannheim (Meylan, France) and phycoerythrin-conjugated anti-TNF-R1 (anti-TNF-R1-PE) and anti-TNF-R2-PE mAbs (both mouse IgG1) from R & D Systems (Minneapolis, Minnesota, USA). The endotoxin content of recombinant IL-6 and TNF-α was less than 2 ng/mg of protein. The B-E8 mAb to IL-6 (mouse IgG1) was kindly provided by Dr Wijdenes (Diaclone, Besançon, France). The A1 neutralizing anti-gp130 mAb (mouse IgG1) was obtained in our laboratory (16). The G4 non neutralizing anti-gp130 mAb (mouse IgG1) was used as control mAb (16).
Human myeloma-cell lines
XG-1, XG-2, XG-4 and XG-6 human myeloma cell lines (HMCL) were obtained from freshly-isolated myeloma cells from four patients with terminal disease as described (6). These HMCL have the same phenotype and the same immunoglobulin gene rearrangements as patients’ freshly-isolated myeloma cells (6). The survival and growth of these cell lines are completely dependent upon addition of exogenous IL-6. The cell lines were cultured in the presence of 1 ng of recombinant human IL-6 in RPMI 1640 supplemented with 10% fetal calf serum (FCS) and 5 × 10−5 M 2-mercaptoethanol (2-ME). Cells were free of mycoplasma contamination.
Assays for detection of apoptotic cells
Two assays were used for detection of apoptotic cells: DMA staining with propidium iodide (PI) or annexin V staining. PI-DNA staining was realized as described by Collins et al. (17). Cells (106 per sample) were washed in PBS, resuspended in 1 ml of 75 % ethanol/25 % water at room temperature for 2 minutes and washed again. Five hundred μl of PBS containing PI (40 μg/ml) and RNAse (100 μg/ml) (both from Sigma Chemical Co.) were added per sample. Cells were incubated for 30 minutes at 37°C and stored at 4°C in the dark before analysis with a FACScan flow cytometer using Cell Quest software (Beckton Dickinson, Moutain View, CA). The cell cycle was analysed with the ModFit LT software (Verity Software House, Topsham, ME). The apoptotic cell fraction appeared in the sub-G1 peak (17). Apoptotic cells were also detected by using fluorescein isothiocyanate-labelled annexin V (FITC-annexin-V, Boehringer Mannheim). Annexin V has a high affinity for phosphatidylserine present on the outer cytoplasmic membrane of apoptotic cells (18). Cells were washed, labelled with Annexin-V-Fluos according the manufacturer’s recommendations and analysed by flow cytometry.
Immunofluorescence staining
Myeloma cells were labelled with either anti-TNF-R1-PE or anti-TNF-R2-PE mAbs and immunofluorescence was analyzed with a FACScan flow cytometer using Cell Quest software (Beckton Dickinson). PE-conjugated mouse IgG1 (Immunotech, Marseilles, France) was used as negative control.
Proliferation assay of HMCL
The cells were washed twice, incubated in RPMI 1640 with 10% FCS for 4 hours at 37°C and washed again. Cells were then cultured for 5 days in 96-well flat-bottomed microtiter plates at 104 cells/well in 200 μl of RPMI 1640 and 10% FCS, with various concentrations of the recombinant cytokines to be tested. Cultures were made in sextuplet. Eight hours before ending the culture, 0.5 μCi per well of [3H]-thymidine (specific activity: 25 Ci/mM, ICN France, Orsay, France) was added and the [3H]-thymidine incorporation determined as previously described (19).
Measurement of lL-6
The HMCL were cultured for 48 hours at a concentration of 106 cells per ml in RPMI1640, 10% PCS in the presence or not of 100 U/ml of TNF-α. Supernatants were harvested and IL-6 was assayed using the IL-6-dependent B9 hybridoma as described (3,20) or using a commercially available IL-6 ELISA (Innotest, Besançon, France). The sensitivities of the bioassay or the ELISA test were similar (about 2 pg/ml).
Statistical analysis
For each culture group, data were mean ± SD of the [3H]-thymidine incorporation determined in six replicate cultures. The statistical significance of these results was determined by using a Student’s t-test for small samples. The statistical significance of the variation of the percentages of apoptotic cells was evaluated by using Student’s t-test for pairs.
RESULTS
TNF-α is a survival factor for cytokine-dependent myeloma cell lines
To investigate the effect of TNF-α on myeloma cell survival, we used cell lines whose survival and proliferation are dependent on addition of exogenous IL-6. As we have previously described (21), upon removal of IL-6, myeloma cells from HMCL rapidly ceased to proliferate and underwent apoptosis. A maximum apoptosis with a limited necrosis was found on day 3 after IL-6 starvation. Thus, in the following experiments, apoptosis was studied on day 3 after IL-6 removal as illustrated in Figure 1 for the XG-6 cell line. Apoptosis was evaluated by quantification of DMA content with PI labelling (less than in G1 phase) or by annexin V staining. The second methodology makes it possible to label apoptotic cells earlier, in agreement with a previous report (18). Upon removal of IL-6, 49 % of the XG-6 cells died by apoptosis within 3 days (mean of 8 separate experiments, Table 1) and TNF-α significantly reduced by 30 % the maximum of apoptosis (Table 1 and Figure 1), although the survival activity of TNF-α was weaker than that of IL-6. This effect was specific to TNF-α as it was inhibited by neutralizing antibodies to TNF-α (results not shown). Similar results were found for 3 out of 4 IL-6-dependent HMCL in at least 5 separate experiments as illustrated in Table 2. The XG-2 HMCL was insensitive to TNF-α. However, this lack of sensitivity was not due to a defect in TNF-R expression because XG-2 cells, as the others XG-cells, expressed marked levels of TNF-R2 on cell surface but no detectable TNF-R1 (results not shown). In what follows, only XG-1, XG-4 and XG-6 will be studied. A maximal survival activity was obtained with 100 U/ml of TNF-α (data not shown). As IL-6, and, more generally the gp130-activating cytokines, are major myeloma cell survival factors, we wanted to see whether the TNF-α survival activity could be mediated by an autocrine gp130 activation. Neutralizing antibodies to gp130 IL-6 transducer or to IL-6 failed to affect TNF-α-induced survival whereas the same antibodies completely inhibited the survival activity of IL-6 (Figure 2). Moreover by using the IL-6-dependent B9 hybridoma as a bioassay (3,20) or an IL-6 ELISA with a sensitivity of 1 pg/ml, we failed to detect any induction of IL-6 production by TNF-α in culture supernatants of HMCL (results not shown). These results indicate that the myeloma cell survival activity of TNF-α was independent of IL-6 or gp130 transducer activation.
Figure 1. TNF-α is a survival factor for human myeloma cells.
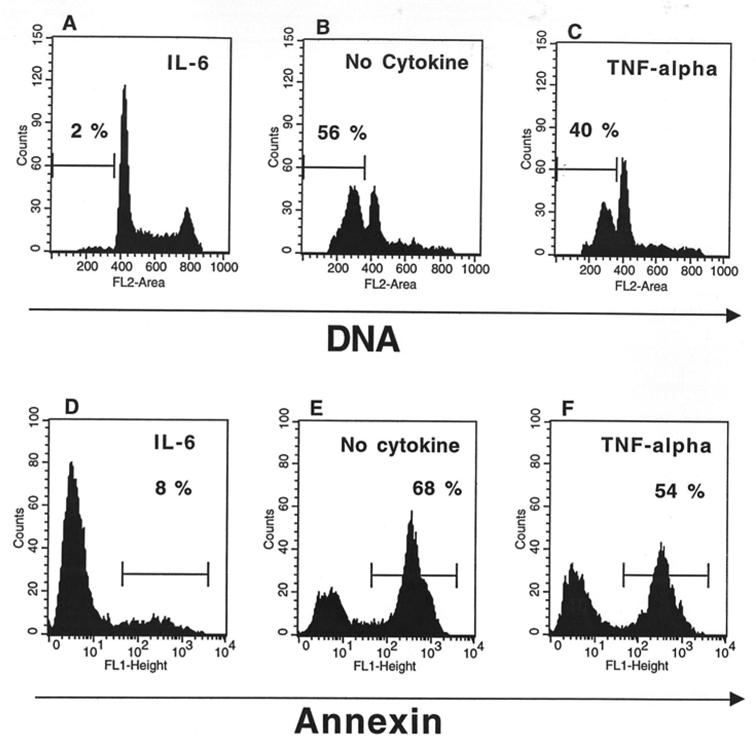
XG-6 cells were extensively washed and cultured at 105 cells per ml for 3 days with either 1 ng/ml of IL-6 (A, D), no cytokine (B, E) or 100 U/ml of TNF-α (C, F). At the end of culture, cells were either stained for DMA with PI (A,B,C) or labelled with FITC-annexin V (D,E,F). The percentage of apoptotic cells (DMA content less than G1 or annexin V positive cells) is indicated in the panels. The fluorescence was analysed with a FACScan apparatus.
Table 1.
TNF-α is a survival factor for XG-6 HMCL
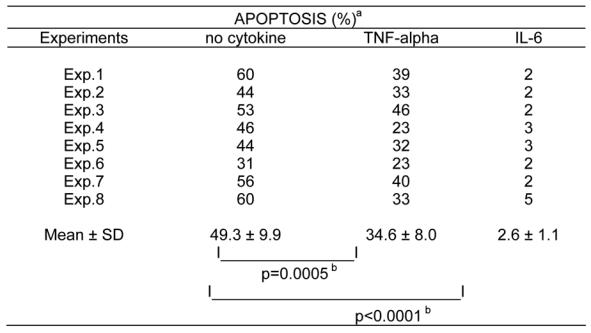
XG-6 cells were cultured for 3 days without cytokine, with 100 U/ml of TNF-α or with 1 ng/ml of IL-6 and apoptosis was assayed by staining DMA with PI.
Apoptosis percentages in each group were compared using a Student’s t-test for pairs and were found to be statistically significant.
Table 2.
TNF-α is a survival factor for 3 out of 4 HMCL
| DNA APOPTOTIC %a |
|||||
|---|---|---|---|---|---|
| HMCL | No Cytokine | TNF-α | IL-6 | nb | pc |
| XG-1 | 22.2 ± 5.5 | 14.1 ± 5.3 | 3.5 ± 1.1 | 10 | 0,0001 |
| XG-4 | 73.3 ± 20.2 | 61.0 ± 21.7 | 7.7 ± 0.6 | 5 | 0,0051 |
| XG-6 | 49.3 ± 9.9 | 33.6 ± 8.0 | 2.6 ± 1.1 | 8 | 0,0005 |
| XG-2 | 12.3 ± 7.5 | 11.7 ± 6.3 | 3.3 ± 1.1 | 5 | nsd |
HMCL were cultured for 3 days with no cytokine, with 100 U/ml of TNF-α or with 1 ng/ml of IL-6 and apoptosis was assayed by staining DNA with PI.
n is the number of experiments.
Apoptosis percentages without cytokines versus TNF-α were compared using a Student’s t-test for pairs and were found to be statistically significant.
not significant.
Figure 2. The survival activity of TNF-α. was not affected by anti-IL-6 or anti-gp130 neutralizing mAbs.
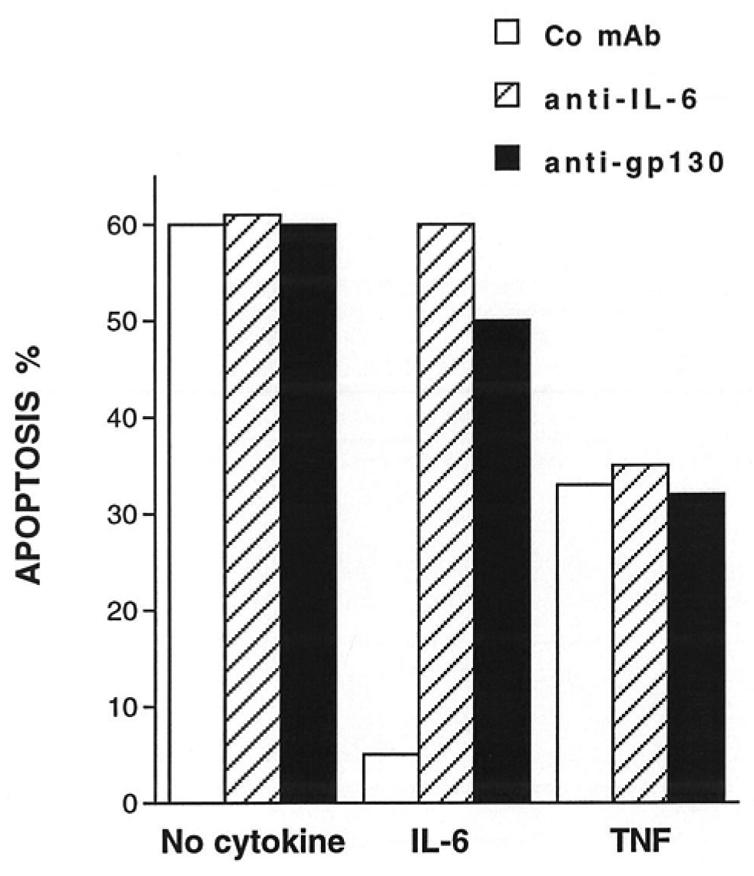
The HMCL XG-6 was cultured for 72 hours at a concentration of 105 cells/ml in culture medium supplemented with 10% of FCS either with IL-6 (1 ng/ml) or TNF-α (100 U/ml) or without exogenous cytokines. Each group was cultured in the presence of either the non-neutralizing anti-gp130 mAb G4 as control (150 μg/ml), or the anti-IL-6 mAb B-E8 (10 μg/ml), or the neutralizing anti-gp130 mAb A1 (150 μg/ml). Apoptosis was assessed by PI DMA staining and analysed by flow cytometry. Results are those of one experiment representative of four separate experiments.
TNF-α is a proliferation and growth factor for cytokine-dependent myeloma cell lines
Upon removal of IL-6, the XG myeloma cells progressively died and the remaining viable cells were partially blocked in the G1 phase of the cell cycle. This is illustrated in Figure 3 for the XG-1 cell line. Addition of TNF-α not only reduced apoptosis but also increased the percentage of viable cells in the S and G2/M phases of the cell cycle for the 3 XG cell lines. Results for XG-1 cells are shown in Figure 3. This finding was confirmed for the 3 XG HMCL by using tritiated thymidine incorporation (Figure 4). As for the survival activity, the maximal proliferation was obtained with 100 U/ml of TNF-α (Figure 4), and it was blocked by an anti-TNF-α mAb but was not affected by an anti-IL-6 mAb (Figure 5). Although the survival and proliferation activities of TNF-α were weaker compared with those of IL-6, TNF-α supported the long-term growth of the XG-1 cytokine-dependent cell line. This is illustrated in Figure 6. Upon removal of IL-6, XG-1 cells progressively died whereas in the presence of IL-6, an exponential growth occurred. TNF-α was able to promote the survival of IL-6-deprived myeloma cells and the long-term growth of these cells in agreement with its survival and proliferation factor activities (Figure 6).
Figure 3. TNF-α is a growth factor for human myeloma cells.
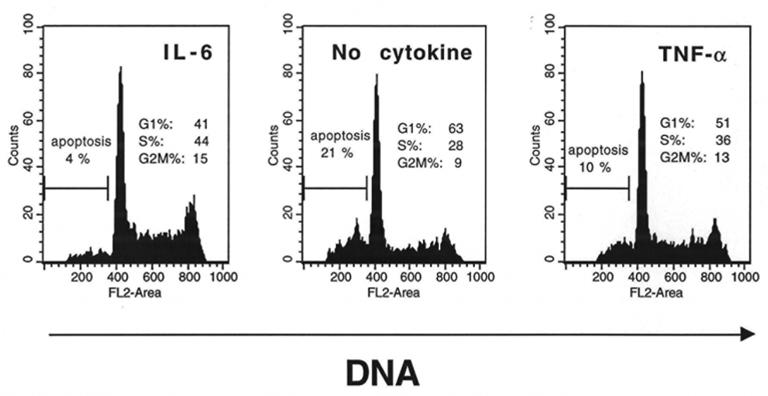
XG-1 cells were extensively washed and cultured at 105 cells per ml for 3 days with either 1 ng/ml of IL-6, or no cytokine or 100 U/ml of TNF-α. At the end of culture, cells were stained for DMA with PI. The percentages of apoptotic cells (DMA content less than G1) and viable cells in the different phases of the cell cycle were determinated using the ModFitLT software. The fluorescence was analysed with a FACScan apparatus. Results are those of one experiment representative of four separate experiments.
Figure 4. TNF-α stimulates the proliferation of IL-6-dependent HMCL.
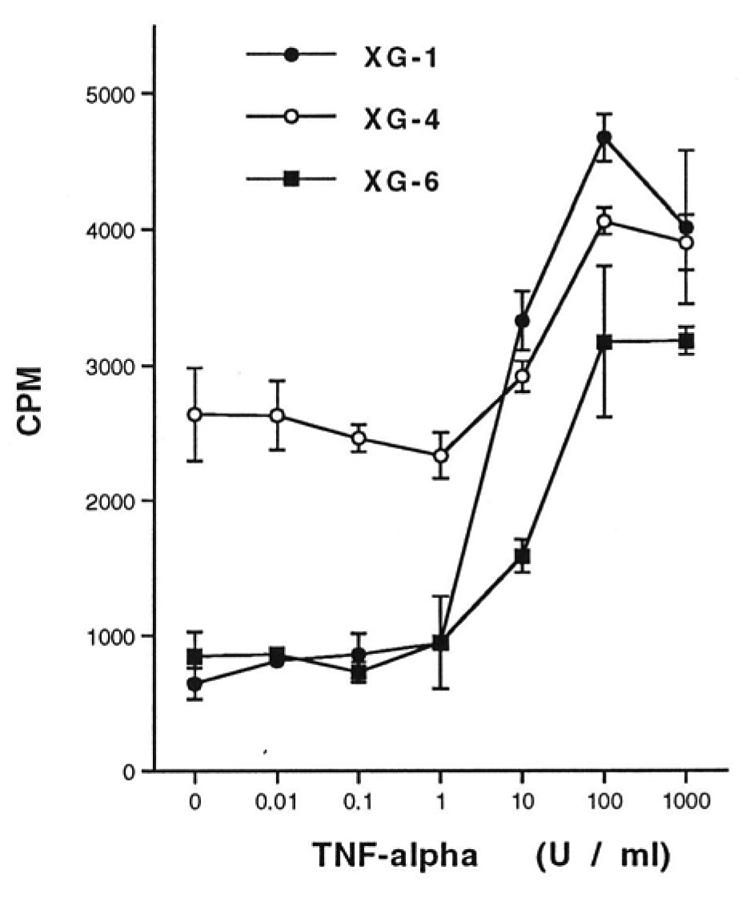
Proliferative response to TNF-α of the HMCL. Results are the mean [3H]-thymidine incorporation ± SD determined on sextuplet culture wells of a representative experiment. These mean values were compared by using a Student’s t test for small samples. For the 3 HMCL (XG-1, XG-4, XG-6), TNF-α induced a significant increase in the mean [3H]-thymidine incorporation (p < 0.001).
Figure 5. The stimulatory effect of TNF-α is specific and is not affected by an anti-IL-6 mAb.
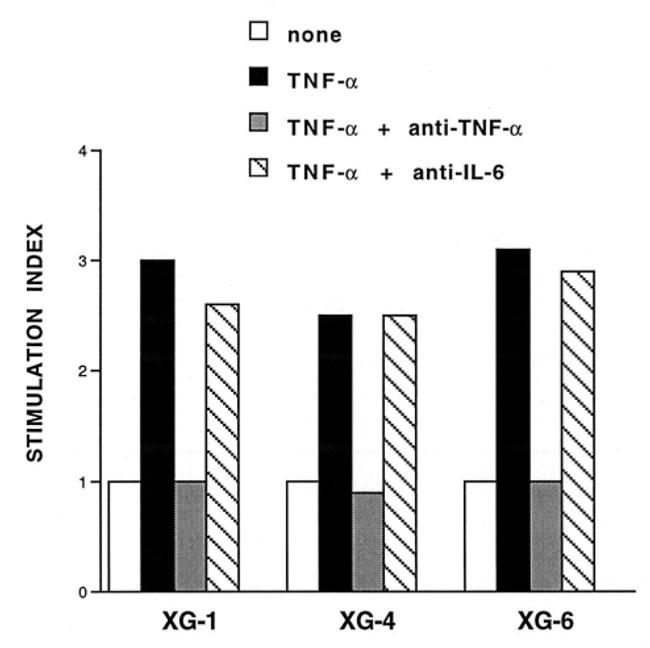
The proliferation of the HMCL was measured by [3H]-thymidine incorporation as described in Materials and Methods. TNF-α was incubated for 2 hours at room temperature with an anti-TNF-α mAb (10 μg/ml) before being added to the cultures. The stimulation indexes are the ratios between the mean [3H]-thymidine incorporation obtained with rTNF-α (100 U/ml), in the presence or not of antibodies, and the mean [3H]-thymidine incorporation obtained with the control medium (1858 ± 120 cpm for XG-1, 2226 ± 298 cpm for XG-4 and 808 ± 56 cpm for XG-6). The anti-IL-6 mAb B-E8 was used at a final concentration of 10 μg/ml. Cultures were made in sextuplet and significance of the results was studied by a Student’s t test for small samples.
Figure 6. TNF-α makes possible the long-term growth of the XG-1 cells without adding exogenous IL-6.
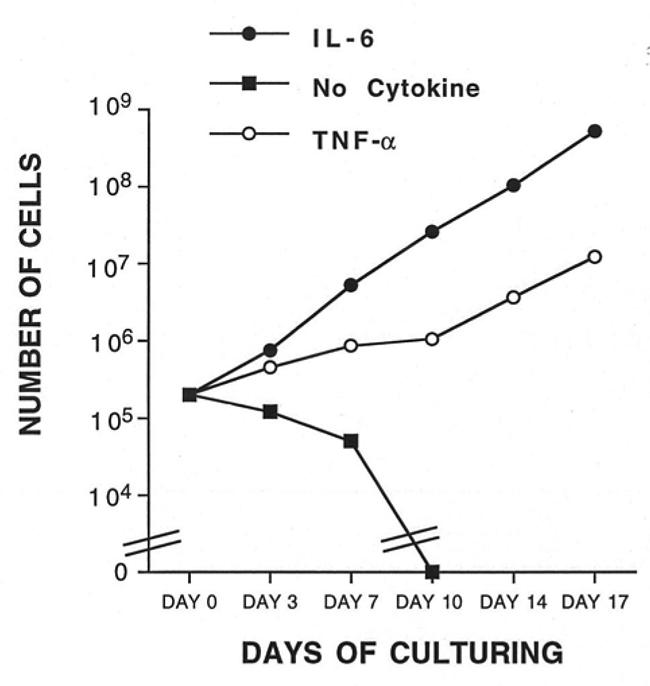
XG-1 cells were extensively washed in order to remove exogenous IL-6 and were cultured at a concentration of 2 × 105 cells/ml in culture medium supplemented with 10% FCS either with IL-6 (1 ng/ml) or TNF-α (100 U/ml) or without exogenous cytokines. Every 4 days, XG-1 cells were reseeded at the initial cell concentration with the respective fresh cytokine combinations. Results are the calculated total number of cells generated from the initial cell input (3 × 105 cells/ml).
DISCUSSION
The current data indicate that TNF-α is not an apoptotic but a survival factor of malignant plasma cell lines. TNF-α is also able to induce myeloma cells in the cell cycle. As TNF-α is an inducer of IL-6 production in various cell types (22–24), we looked to see whether this activity was mediated through a gp130 cytokine. Our results indicated that it was not the case. First, we failed to find any IL-6 production induced by TNF-α in myeloma cell lines. Secondly, the survival and proliferation activities induced by TNF-α were not affected by the anti-IL-6 and anti-gp130 mAb that completely inhibited the activity of gp130 cytokines (16). It would be interesting to identify whether TNF-α and IL-6 might share some signalling pathways leading to myeloma cell survival and proliferation. As indicated above, the anti-apoptotic activity of TNF-α is related to NF-kappa B activation (13–15). The transduction pathways involved in IL-6 survival activity has not yet been identified whereas the IL-6 proliferation activity involves the Ras/MAP kinase pathway (25). TNF was recently shown to activate the JAK/STAT pathway as IL-6 (26) and IL-6 activates various signalling pathways but, in particular, STATS that may be involved in NF-kappa B activation (27) and eventually, in the antiapoptotic activity.
Several reports have shown that TNF-α is produced in tumoral samples of patients with multiple myeloma (28,29) and that this cytokine could contribute to the endogenous IL-6 production by the tumoral environment (28,30). In addition, serum levels of TNF-α have been shown to be increased in patients with active MM compared with normal controls (31) and to be associated with a poor prognosis (32). In this disease, IL-6 is the major survival and proliferation factor (4). Thus, these two activities of TNF-α, i.e. induction of IL-6 production by the tumoral environment and survival activity of tumoral plasma cells suggest that TNF antagonists, that are already available at a clinical level, might be useful for treating patients with MM, possibly in association with other anti-inflammatory agents such as corticosteroids.
References
- 1.Hardin J, MacLeod S, Grigorieva I, Chang R, Barlogie B, Xiao H, Epstein J. Interleukin-6 prevents dexamethasone-induced myeloma cell death. Blood. 1994;84:3063. [PubMed] [Google Scholar]
- 2.Kawano M, Hirano T, Matsuda T, Taga T, Horii Y, Iwato K, Asaoka H, Tang B, Tanabe O, Tanaka H, Kuramoto A, Kishimoto T. Autocrine generation and essential requirement of BSF-2/IL-6 for human multiple myeloma. Nature. 1988;332:83. doi: 10.1038/332083a0. [DOI] [PubMed] [Google Scholar]
- 3.Klein B, Zhang XG, Jourdan M, Content J, Houssiau F, Aarden L, Piechaczyk M, Bataille R. Paracrine rather than autocrine regulation of myeloma-cell growth and differentiation by interleukin-6. Blood. 1989;73:517. [PubMed] [Google Scholar]
- 4.Klein B, Zhang XG, Lu ZY, Bataille R. Interleukin-6 in human multiple myeloma. Blood. 1995;85:863. [PubMed] [Google Scholar]
- 5.Bataille R, Harousseau JL. Multiple myeloma. N Engl J Med. 1997;336:1657. doi: 10.1056/NEJM199706053362307. [DOI] [PubMed] [Google Scholar]
- 6.Zhang XG, Gaillard JP, Robillard N, Lu ZY, Gu ZJ, Jourdan M, Boiron JM, Bataille R, Klein B. Reproducible obtaining of human myeloma cell lines as a model for tumor stem cell study in human multiple myeloma. Blood. 1994;83:3654. [PubMed] [Google Scholar]
- 7.Shimizu S, Yoshioka R, Hirose Y, Susumu S, Tachibana J, Konda S. Establishment of two interleukin-6 (B cell stimulatory factor 2/interferon β2) dependent human bone marrow-derived myeloma cell lines. J Exp Med. 1989;169:339. doi: 10.1084/jem.169.1.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Westendorf JJ, Ahmann GJ, Greipp PR, Witzig TE, Lust JA, Jelinek DF. Establishment and characterization of three myeloma cell lines that demonstrate variable cytokine responses and abilities to produce autocrine interleukin-6. Leukemia. 1996;10:866. [PubMed] [Google Scholar]
- 9.Borset M, Waage A, Brekke OL, Helseth E. TNF and IL-6 are potent growth factors for OH-2, a novel human myeloma cell line. Eur J Haematol. 1994;53:31. doi: 10.1111/j.1600-0609.1994.tb00176.x. [DOI] [PubMed] [Google Scholar]
- 10.Baker SJ, Reddy EP. Transducers of life and death: TNF receptor superfamily and associated proteins. Oncogene. 1996;12:1. [PubMed] [Google Scholar]
- 11.Nagata S. Apoptosis by death factor. Cell. 1997;88:355. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 12.Malinin NL, Boldin MP, Kovalenko AV, Wallach D. MAP3K-related kinase involved in NF-kappaB induction by TNF, CD95 and IL-1. Nature. 1997;385:540. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 13.Wang CY, Mayo MW, Baldwin AS., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274:784. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 14.Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274:787. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 15.Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 16.Liautard J, Sun RX, Cotte N, Gaillard JP, Mani JC, Klein B, Brochier J. Specific inhibition of IL-6 signalling with monoclonal antibodies against the gp130 receptor. Cytokine. 1997;9:233. doi: 10.1006/cyto.1996.0159. [DOI] [PubMed] [Google Scholar]
- 17.Collins MKL, Marvel J, Malde P, Lopez-Rivas A. Interleukin-3 protects murine bone marrow cells from apoptosis induced by DMA damaging agents. J Exp Med. 1992;176:1043. doi: 10.1084/jem.176.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 19.Jourdan M, Zhang XG, Portier M, Boiron JM, Bataille R, Klein B. IFN-alpha induced autocrine production of IL-6 in myeloma cell lines. J Immunol. 1991;147:4402. [PubMed] [Google Scholar]
- 20.Helle M, Boeise L, Aarden LA. Functional discrimination between interleukin-6 and interleukin-1. Eur J Immunol. 1988;18:1535. doi: 10.1002/eji.1830181010. [DOI] [PubMed] [Google Scholar]
- 21.Jourdan M, Ferlin M, Legouffe E, Horvathova M, Liautard J, Rossi JF, Wijdenes J, Brochier J, Klein B. The myeloma cell antigen syndecan-1 is lost by apoptotic myeloma cells. Br J Haematol. 1998;100:637. doi: 10.1046/j.1365-2141.1998.00623.x. [DOI] [PubMed] [Google Scholar]
- 22.Kohase M, Henriksen-DeStefano D, May LT, Vilcek J, Sehgal PB. Induction of beta 2-interferon by tumor necrosis factor: a homeostatic mechanism in the control of cell proliferation. Cell. 1986;45:659. doi: 10.1016/0092-8674(86)90780-4. [DOI] [PubMed] [Google Scholar]
- 23.Van Damme J, Opdenakker G, Simpson RJ, Rubira MR, Cayphas S, Vink A, Billiau A, Van Snick J. Identification of the human 26-kD protein, interferon beta 2 (IFN-beta 2), as a B cell hybridoma/plasmacytoma growth factor induced by interleukin-1 and tumor necrosis factor. J Exp Med. 1987;165:914. doi: 10.1084/jem.165.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirano T. Interleukin 6 (IL-6) and its receptor: their role in plasma cell neoplasias. Int J Cell Cloning. 1991;9:166. doi: 10.1002/stem.5530090303. [DOI] [PubMed] [Google Scholar]
- 25.Ogata A, Chauhan D, Teoh G, Treon SP, Urashima M, Schlossman RL, Anderson KC. IL-6 triggers cell growth via the Ras-dependent mitogen-activated protein kinase cascade. J Immunol. 1997;159:2212. [PubMed] [Google Scholar]
- 26.Guo D, Dunbar JD, Yang CH, Pfeffer LM, Donner DB. Induction of Jak/STAT signaling by activation of the type 1 TNF receptor. J Immunol. 1998;160:2742. [PubMed] [Google Scholar]
- 27.Yang CH, Murti A, Pfeffer LM. STATS complements defects in an interferon-resistant cell line: evidence for an essential role for STAT3 in interferon signaling and biological activities. Proc Natl Acad Sci U S A. 1998;95:5568. doi: 10.1073/pnas.95.10.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lichtenstein A, Berenson J, Norman D, Chang MP, Carlile A. Production of cytokines by bone marrow cells obtained from patients with multiple myeloma. Blood. 1989;74:1266. [PubMed] [Google Scholar]
- 29.Cozzolino F, Torcia M, Aldinucci D, Rubartelli A, Miliani A, Shaw AR, Lansdorp PM, Di Gugliemo R. Production of interleukin-1 by bone marrow myeloma cells. Blood. 1989;74:380. [PubMed] [Google Scholar]
- 30.Carter A, Merchav S, Silvian-Draxler I, Tatarsky I. The role of interleukin-1 and tumor necrosis factor-α in human multiple myeloma. Br J Haematol. 1990;74:424. doi: 10.1111/j.1365-2141.1990.tb06330.x. [DOI] [PubMed] [Google Scholar]
- 31.Filella X, Blade J, Guillermo AL, Molina R, Rozman C, Ballesta AM. Cytokines (IL-6, TNF-alpha, IL-1 alpha) and soluble interleukin-2 receptor as serum tumor markers in multiple myeloma. Cancer Detect Prev. 1996;20:52. [PubMed] [Google Scholar]
- 32.Blade J, Filella X, Montoto S, Bosch F, Molina R, Coca F, Lopez-Guillermo A, Cid J, Ballesta AM, Montserrat E. Clinical relevance of interleukine-6 and tumor necrosis factor alpha serum levels in monoclonal gammopathy of undetermined signifiance. Blood. 1997;90:351a. (Abstract) [Google Scholar]


