Abstract
A trans-dominant mutational strategy was used to down-regulate trypanothione reductase (TR) activity levels in Leishmania donovani, the causative agent of visceral leishmaniasis in humans. TR, regarded as an ideal drug target against trypanosomatid infections, is a homodimeric flavoprotein oxidoreductase unique to these organisms that plays a central role in the enzymatic regeneration of the thiol pool. Extrachromosomal, heterologous expression of a trans-dominant mutant version of the Trypanosoma cruzi enzyme in L. donovani resulted in the formation of inactive cross-species heterodimers and in a dramatic decrease of endogenous TR activity levels. Recombinant cells depleted of up to 85% of TR activity were significantly impaired in their ability to regenerate dihydrotrypanothione from trypanothione disulfide following oxidation with diamide. Nonetheless trans-dominant mutant recombinants were still capable of maintaining a reduced intracellular environment during cell growth in culture and were able to metabolize hydrogen peroxide at wild-type rates in vitro. Importantly, however, cells expressing the trans-dominant mutant enzyme displayed a decreased ability to survive inside activated macrophages in a murine model of Leishmania infection. The apparent inability of Leishmania to modulate the expression of active TR homodimers in response to the expression of trans-dominant mutant protein suggests that specific inhibitors of this enzyme should be useful anti-leishmanial agents.
Trypanosomatid protozoa are members of the order Kinetoplastida that includes parasites of relevant medical and veterinary importance such as Leishmania spp. and Trypanosoma spp. Their complex life cycles involve alternation between an insect vector and a vertebrate host and entail dramatic morphological changes that are developmentally regulated (1). In susceptible hosts trypanosomatids can cause severe illness and in some cases death. Adequate protective vaccines against trypanosomatid infections have yet to be developed, and drugs currently available for chemotherapeutic intervention are mostly unsatisfactory mainly because of their lack of specificity, toxicity to humans, and, in many cases, to developed parasite resistance (2).
The search for parasite-specific, physiologically relevant cellular components that may be developed as drug targets and their validation by genetic means are the fundamentals of a rational approach to the design and discovery of drugs against trypanosomatid infections. The trypanothione system of trypanosomatid protozoa, a unique thiol-redox cycling system that protects the parasite against damage by oxidants and toxic heavy metals, represents a promising source of unique metabolites and enzymatic activities with potential as antiparasitic drug targets. Although analogous to the glutathione system that operates in humans and almost all other aerobic organisms the trypanothione system offers a number of distinct features that may be exploited for selective attack. One such example is the pronounced difference in substrate specificity between trypanothione reductase (TR) and its mammalian counterpart, glutathione reductase (reviewed in ref. 3). The important role of TR in thiol metabolism and its absence from human cells suggests that it may be ideally suited for development as a chemotherapeutic target. Attempts to validate this oxidoreductase as a drug target by molecular genetics and biochemical means have been reported recently (4–6). Biochemical studies involving the overexpression of Leishmania TR in Leishmania donovani and Trypanosoma cruzi demonstrated that regeneration of dihydrotrypanothione (T(SH)2) from trypanothione disulfide (T(S)2) is neither limiting in the management of oxidative stress thought to be induced by antiparasitic drugs such as nifurtimox, nitrofurazone, and gentian violet, nor in the metabolism of hydrogen peroxide (H2O2), one of the reactive oxygen species toxic to Leishmania (4, 7, 8). Attempts to down-regulate TR activity by antisense RNA expression in T. cruzi failed to decrease endogenous activity levels apparently as a result of a specific sequence inversion in a proportion of antisense plasmid DNA molecules (5). Gene targeting experiments have also failed to produce null mutants completely devoid of TR catalytic activity (ref. 6; J.T. and A.H.F., unpublished data).
Evaluation of the minimal levels of TR activity required for parasite survival is fundamental for the purpose of inhibitor design. Recently we created by site-directed mutagenesis a set of negative-complementing TR mutants that retain their natural folding and dimerization properties but which are devoid of catalytic activity (ref. 9; unpublished data). We have now used these mutants to generate a trans-dominant mutant version of the T. cruzi TR enzyme. Here we report on its heterologous expression in L. donovani and on some of the phenotypic consequences of the stepwise inactivation of endogenous TR catalytic activity in Leishmania.
MATERIALS AND METHODS
Parasites.
A cloned promastigote line of L. donovani (MHOM/ET/67/HU3) was grown at 22–24°C in M199 medium (GIBCO/BRL) supplemented with 40 mM Hepes (pH 7.4), 0.1 mM adenine, 7.7 mM haemin, 10% (vol/vol) heat inactivated fetal calf serum, 50 units/ml penicillin, and 50 μg/ml streptomycin. Cells were maintained by subculture and kept at densities ranging between 5 × 105 to 3 × 107 cells ml−1.
Transfection of Parasites.
Transfection of L. donovani was performed essentially as described (4). Late-log phase promastigotes were electroporated in the presence or absence of 25 or 50 μg of supercoiled plasmid DNA. Cells were diluted 2-fold with culture medium and allowed to recover for 24 h at 24°C. Recombinant clones were selected on M199 medium solidified with 1% bactoagar and supplemented with 25 μg/ml G418.
Plasmid Construction.
Plasmid pC53A harbors a mutant version of the T. cruzi TR-encoding gene (tryA) in which the essential redox-active cysteine at position 53 has been replaced by an alanine residue (9). This plasmid was used as a template to introduce a second point mutation that changed the essential active site histidine at position 461 by a glutamine residue by using mutagenic oligonucleotide TcTRHQ (5′-ATTGGTGTGCAgCCCACAAGT-3′). The presence of both mutations in the resulting plasmid pC53A/H461Q was confirmed by sequencing the entire tryA coding region. This gene was subsequently cloned into the EcoRV site of expression vector pTEX (10) by conventional methodology (11) to generate plasmid pTEXTcTRtdm.
Enzymatic Assays.
TR and alanine aminotransferase (ALAT) activities were assayed in cell-free extracts prepared as described (5) by using a Beckman DU-70 spectrophotometer fitted with a cell temperature regulator. TR reactions were monitored spectrophotometrically by following the T(S)2-dependant oxidation of NADPH at 340 nm (12). ALAT reactions also were monitored at 340 nm following the pyruvate-dependent oxidation of NADH by lactate dehydrogenase coupled to the ALAT-dependant synthesis of pyruvate from l-alanine and α-ketoglutarate as described (5). One unit of ALAT or TR activity is defined as the amount of enzyme required to oxidize 1 μmol of NADH or NADPH (respectively) per minute at 27°C. Protein concentrations were determined by the method of Bradford using BSA as a standard (13).
Analysis of Heterodimer Formation.
Cultures (100 ml) of L. donovani or T. cruzi transfected with either pTEXLdTR (14) or pTEXTcTR (5) were harvested and TR extracted as described (15). Following treatment with protamine sulfate and dialysis (14), each extract (0.25 mg protein) was analyzed by fast protein liquid chromatography (FPLC) on an anion exchange Mono Q Sepharose column (Pharmacia). TR was eluted with a linear gradient of KCl (0–0.4 M over 50 min; flow rate 1.0 ml/min) and 1 ml fractions collected for analysis.
DNA Manipulations.
Total DNA was prepared by using a mini-prep procedure (16). For DNA blot analysis, 2 μg aliquots were digested with appropriate restriction enzymes, electrophoresed, and blotted onto nylon membranes (Hybond N; Amersham). Hybridization to probes labeled to high specific activity by random priming (17) was carried out by using standard techniques (11).
Determination of Thiol Content.
Monobromobimane-derivatized thiols were analyzed by HPLC as described (18). Thiol regeneration following diamide oxidation was assessed in cells prepared and processed exactly as described (4).
H2O2 Toxicity and Metabolism.
Toxicity of H2O2 to Leishmania was assessed by monitoring growth inhibition as follows: cells were seeded at a density of 5 × 105 ml−1 in culture well plates containing G418 (70 μg/ml) and increasing concentrations of H2O2 and incubated at 24°C until controls reached a cell density between 1.5 and 2 × 107 ml−1 (4–5 days). IC50 values were determined by counting cells in a haemocytometer and plotting the percentage of growth inhibition relative to control cells against H2O2 concentration. Metabolism of H2O2 was monitored essentially as described (19) by using 1 × 107 cells ml−1 and an initial H2O2 concentration of 40 μM; incubations were carried out at 22°C.
Cell Cultures and Infectivity Assays.
Bone marrow-derived macrophages were obtained from 6- to 7-week-old female BALB/c mice (Charles River) as described (20). Macrophages were rested for 2 days in the absence of colony stimulating factor 1, harvested, and seeded into 16-chamber tissue culture slides (Nunc). Bone marrow-derived macrophages were infected at 37°C with stationary-phase promastigotes of wild-type and recombinant L. donovani at a 1:20 cell-to-parasite ratio. After 4 h, excess parasites were eliminated by vigorous washing with serum-free DMEM. Infected macrophages were activated with 11 units/ml murine recombinant interferon-γ (IFN-γ; Genentech) in DMEM supplemented with 10% heat-inactivated fetal calf serum and 10 ng/ml lipopolysaccharide (LPS; Sigma). After incubation periods of 4, 24, 48, and 72 h, cultures were fixed in methanol, stained with Giemsa, and examined microscopically.
RESULTS
Rational and Construction of Recombinant Clones.
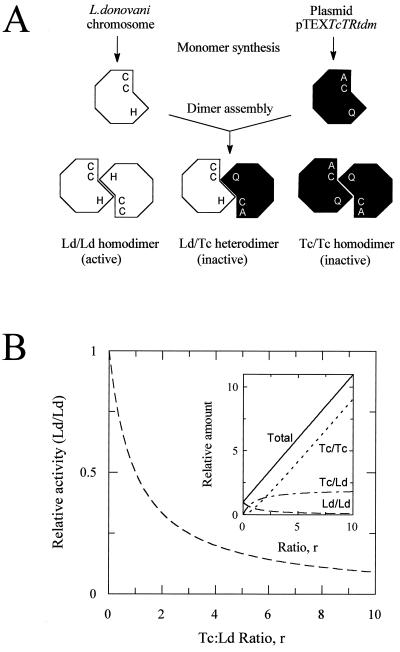
A schematic representation of the strategy used is shown in Fig. 1A. Although TR monomers are catalytically inactive, they readily associate to form fully active homodimers, each containing two active sites. Within each active site three essential amino acid residues have been identified, two cysteines (Cys-53 and Cys-58) that form the redox active center of the enzyme (9) and a histidine (His-461) that acts as the final proton acceptor/donor (M.L.C., J.T., and A.H.F., unpublished data). Because at least one of the essential residues is contributed by each monomer during dimerization, the expression of trans-dominant mutant monomers should lead to the stepwise inactivation of endogenous TR by formation of inactive heterodimers. Assuming that interactions between endogenous monomers (Ld) and mutant monomers (Tc) occur randomly with equal affinity, then the relative distribution of TR dimers will be dependent on the level of overexpression of mutant protein (r) according to the expression (1 + r)2. Because the total monomer concentration will increase with increasing drug selection pressure as a function (1 + r), then the relative amounts of each type of dimer are 1/(1 + r), 2r/(1 + r) and r2/(1 + r) for Ld/Ld, Ld/Tc and Tc/Tc, respectively (Fig. 1B).
Figure 1.
(A) Representation of the trans-dominant mutational strategy used in this study. TR monomers from L. donovani and T. cruzi associated either as homodimers or heterodimers are illustrated showing the essential cysteine and histidine residues that comprise the trypanothione-binding region of the active site. (B) Effect of overexpression of trans-dominant mutants on levels of active enzyme in L. donovani. The extent of inactivation of endogenous TR (Ld/Ld) depends on the level of overexpression of mutant protein (Tc:Ld ratio, r) as described in the text. (Inset) Theoretical relative amounts of each dimer species as a function of heterologous protein overexpression.
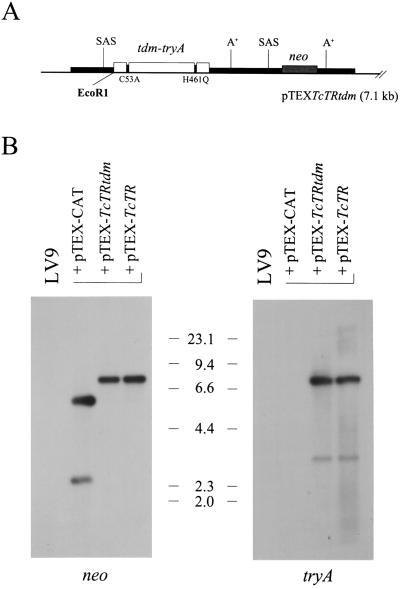
Plasmid pTEXTcTR has been described (5) and was used as an experimental control for TR overexpression. Plasmid pTEXTcTRtdm, constructed as described above, carries a trans-dominant mutant copy of the T. cruzi tryA gene (Fig. 2A). These constructs were transfected into L. donovani, and their structural integrity was analyzed by Southern blotting. As shown in Fig. 2B, both neo and tryA probes identified major bands of the expected size (7.1 kb) for plasmid DNA of monomeric structure. In addition, a smaller and fainter band indicative of truncated plasmid DNA was also detected in each of our recombinant lines; such truncated plasmid molecules have also been observed previously for other pTEX-derived constructs (ref. 10; unpublished observations).
Figure 2.
Analysis of recombinant lines transfected with pTEXTcTRtdm. (A) Structure of expression plasmid. The tryA coding region, the bacterial neomycin phosphotransferase (neo) gene, and GAPDH untranslated regulatory sequences are represented by open, grey, and filled boxes, respectively. The location of polyadenylation (A+) and splice acceptor sites (SAS) within regulatory regions is indicated; also shown is the unique EcoRI restriction site. (B) DNA blot analysis. Total DNA isolated from indicated lines was digested with EcoRI, size-fractionated, and blotted onto nylon; the membrane was sequentially probed with radiolabeled neo and tryA sequences. Sizes of molecular markers are given in kilobases.
Heterodimer Formation in Recombinant Clones.
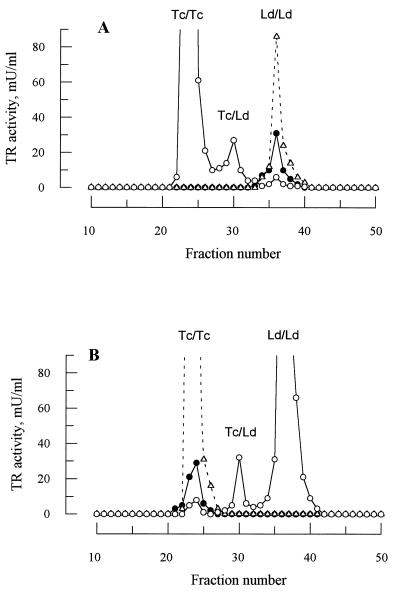
Attempts to produce heterodimers in vitro by mixing purified TR from L. donovani and T. cruzi under a variety of native and denaturing conditions proved unsuccessful (data not shown). Thus, to determine whether heterodimer formation between LdTR and TcTR occurs in vivo, equal amounts of cell extract from lines of L. donovani and T. cruzi expressing either homologous or heterologous wild-type TR from plasmid pTEX were separated by anion exchange chromatography and analyzed for TR activity (Fig. 3). Because TR from each species has a different isoelectric point, separation of the different isoenzymes can be readily achieved by elution with a linear salt gradient. Wild-type L. donovani and cells overexpressing LdTR show a single peak of enzyme activity centered on fraction 36 (Ld/Ld, Fig. 3A). In contrast, overexpression of TcTR in L. donovani is associated with a pronounced reduction in the Ld/Ld peak as well as two additional peaks of TR activity representing the Tc/Tc homodimer and the Tc/Ld heterodimer. The converse result is obtained in T. cruzi (Fig. 3B), confirming that the middle peak centered on fraction 30 represents the heterodimer Tc/Ld. A further analysis of the distribution of TR activity indicates that the concentration of heterodimers is significantly under-represented in both cases compared with the theoretical distribution predicted for the observed 10- to 12-fold overexpression (Table 1). Moreover, in both cases the residual Ld/Ld or Tc/Tc endogenous homodimers represent ≈20% of the wild-type level rather than <10% predicted from Fig. 1B.
Figure 3.
Heterodimer formation in L. donovani and T. cruzi. Cells that had been transfected with a pTEX plasmid containing either homologous or heterologous tryA were analyzed for TR content by anion exchange chromatography as described. (A) L. donovani promastigotes: wild-type control (•), transfected with pTEXTcTR (○), and transfected with pTEXLdTR (▵). (B) T. cruzi epimastigotes: wild-type control (•), transfected with pTEXLdTR (○), ad transfected with pTEXTcTR (▵). The identity of the homodimers (Tc/Tc or Ld/Ld) and heterodimer peaks (Tc/Ld) are indicated.
Table 1.
Distribution of enzyme activity in cell lines overexpressing heterologous functional TR
| Cell type | Distribution | TR activity, mU
|
||||
|---|---|---|---|---|---|---|
| Tc/Tc | Tc/Ld | Ld/Ld | Total | Recovery* | ||
| L. donovani WT | – | – | 66 | 66 | 105 | |
| +pTEXTcTR | O | 747 | 70 | 13 | 830 | 93 |
| E | 737 | 90 | 3 | |||
| (O-E)2/E | 0.14 | 4.62 | 37.7 | 42.4† | ||
| T. cruzi WT | 66 | – | – | 66 | 111 | |
| +pTEXLdTR | O | 14 | 50 | 527 | 591 | 100 |
| E | 3 | 73 | 516 | |||
| (O-E)2/E | 50.7 | 7.17 | 0.25 | 58.1† | ||
The observed distribution of activity (O) is calculated from the experiment shown in Fig. 3. The expected distribution of enzyme activity (E) is calculated from the frequencies of the different monomers (Ld frequency p and Tc frequency q) according to the expression (p + q)2 = 1 (the “Hardy-Weinberg ratio”). WT, wild type.
Recovery of TR activity from the ion exchange column.
The χ2 with one degree of freedom indicates that under-representation of the heterodimers in both recombinant populations is highly significant (P ≪ 0.001).
Enzymatic Phenotype of Clones Expressing Trans-Dominant Mutant TR.
TR and ALAT catalytic activities were measured in cell-free extracts. Cytosolic ALAT was used to assess the general metabolic state of cells and to control for possible variability in extraction or loss in activity caused by proteolytic degradation. In contrast to the 8-fold overexpression of TR activity found in cells harboring plasmid pTEXTcTR, a dramatic decrease in activity levels (75–85% relative to the wild-type control) was observed in six independent L. donovani clones overexpressing the trans-dominant mutant protein (Table 2). No significant difference in ALAT activity was found between untransfected and recombinant cells, suggesting that the decrease in TR activity is a specific consequence of the expression of mutant TR protein. As pTEX-derived vectors are unstable in the absence of selection (10), we further established the specificity of the recombinant phenotype by removing G418 from the culture medium. This removal resulted in the gradual restoration of wild-type TR activity levels in trans-dominant recombinant lines; after 45 generations in the absence of selection, trans-dominant mutant cells fully regained their wild-type levels of TR catalytic activity (data not shown). Control cells that overexpress chloramphenicol acetyltransferase, a neutral protein, showed no significant changes in TR activity levels under the same experimental conditions.
Table 2.
Enzymatic activity in transgenic L. donovani
| Cell type | TR
|
ALAT
|
||
|---|---|---|---|---|
| mU/mg | Rel. value | mU/mg | Rel. value | |
| LV9 WT | 220 ± 12 | 1.00 | 77 ± 03 | 1.00 |
| +pTEXTcTRtdm | ||||
| (1)* | 32 ± 06 | 0.14 | 81 ± 05 | 1.05 |
| (3) | 51 ± 11 | 0.23 | 83 ± 08 | 1.07 |
| (8) | 30 ± 03 | 0.14 | 74 ± 05 | 0.96 |
| (9) | 41 ± 06 | 0.19 | 71 ± 03 | 0.92 |
| (10) | 38 ± 02 | 0.17 | 84 ± 11 | 1.09 |
| (12) | 36 ± 07 | 0.16 | 76 ± 07 | 0.99 |
| +pTEXTcTR | 1,836 ± 113 | 8.35 | 65 ± 04 | 0.84 |
Cells were prepared and analyzed for TR and ALAT activities as described. WT, wild type.
Clone identification number.
Response to Oxidants.
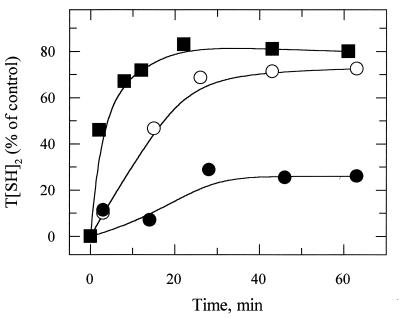
The main physiological role of TR is to regenerate low molecular mass thiols oxidized during aerobic metabolism, so we tested the ability of our recombinant cell lines to regenerate the thiol pool following oxidation with diamide. Trypanothione regeneration over time was monitored upon removal of the agent from the culture medium and was expressed as the percentage of T(SH)2 levels found in untreated cells. Fig. 4 shows that TR activity levels and the rate of T(SH)2 regeneration from T(S)2 are directly correlated. Relative to the wild-type cells, the initial rates of regeneration of T(SH)2 in cells expressing pTEXTcTR and pTEXTcTRtdm were 7.4- and 0.21-fold, respectively, in good agreement with the relative TR activities reported in Table 2. Similar observations were made when regeneration of glutathione, glutathionyl-spermidine, and ovothiol A was monitored (data not shown). When the initial thiol pool sizes of recombinant and wild-type cells were measured in control experiments they were found to be essentially the same, suggesting that under normal conditions of culture TR activity levels found in trans-dominant mutant recombinants are not rate-limiting for the enzymatic regeneration of the thiol pool.
Figure 4.
Trypanothione regeneration following diamide-induced oxidant stress in L. donovani promastigotes. Regeneration of T(SH)2 over time was monitored as described. Plotted values represent the percentage of T(SH)2 found in untreated control cells: wild-type (control line; ○), pTEXTcTRtdm (TR underexpressing line; •), and pTEXTcTR (TR overexpressing line; ▪).
H2O2 has long been implicated in the killing of Leishmania by activated macrophages (6, 7), and the trypanothione system has been proposed to be involved in its detoxification (reviewed in ref. 3). However, as shown in Table 3, the ability of recombinant cells with decreased (trans-dominant mutant expressors) or elevated (TR overexpressors) TR activity to metabolise this compound at rates equivalent to wild-type Leishmania indicate that, under our experimental conditions, TR is not a limiting factor in the removal of H2O2 from these cells.
Table 3.
Hydrogen peroxide metabolism and toxicity to L. donovani
| Cell type | TR mU/mg | H2O2 metabolism, nmol/min/108 cells | IC50, mM |
|---|---|---|---|
| LV9 wild type | 220 | 7.2 ± 0.9 | 0.42 ± 0.08 |
| +pTEXTcTRtdm | 36 | 6.4 ± 1.0 | 0.35 ± 0.06 |
| +pTEXTcTR | 1,836 | 7.0 ± 0.6 | 0.38 ± 0.06 |
Invasion and Survival in Macrophages.
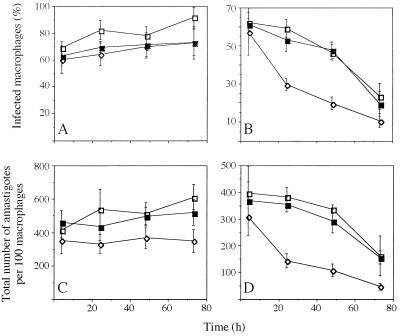
Given the proposed participation of TR in the protection of the cell against toxic free radicals, we tested the ability of recombinant parasites with decreased TR activity levels to invade and survive in resting or activated rodent macrophages. Invasiveness was found not to be affected by the expression of trans-dominant mutant enzyme as similar numbers of wild-type and recombinant Leishmania were phagocytozed by macrophages (Fig. 5). Likewise, parasites with decreased TR activity survived equally well as wild-type cells inside resting macrophages (Fig. 5 A and C). However, following macrophage activation by IFN-γ (11 units/ml) and LPS (10 ng/ml) a significant difference in the rate of parasite clearance was observed between trans-dominant mutant expressors and control cells (Fig. 5 B and D). That this increased susceptibility to macrophage killing was reversed by growing recombinant lines in the absence of the plasmid-selecting drug (G418) prior to exposure to macrophages (Fig. 5 B and D) underscores the specificity of the mutant phenotype and the importance of TR for the survival of the parasite inside its mammalian host.
Figure 5.
Effect of TR down-regulation on L. donovani macrophage colonization. Untreated (A and C) or cytokine-activated macrophages (B and D) were infected as described with L. donovani: wild-type control (□), transfected with pTEXTcTRtdm (⋄), and same recombinant line grown in the absence of plasmid-selection for a minimum of 60 generations (▪). Plotted values represent the percentage of infected macrophages (A and B) and the total number of parasites per 100 macrophages (C and D) as determined from three independent experiments.
DISCUSSION
The stepwise down-regulation of TR with a trans-dominant mutational strategy indicates that as little as 15% of normal TR activity is sufficient to support promastigote growth in culture. The observed levels of catalytic activity down-regulation are consistent with a theoretical relative enzyme ratio in the range 4.3 to 7.3 in recombinant clones harboring pTEXTcTRtdm (Fig. 1B). However, this is likely to be an underestimate of the level of mutant enzyme overexpression because heterodimer formation is disfavored over homodimer formation (Table 1). The reason for the deviation from the distribution predicted by the Hardy–Weinberg equation is not known, but it suggests that one of the original assumptions used in deriving the equation is not valid. Either monomer association is not entirely random or the association constants for heterodimer and homodimer formation are not identical. Attempts to decrease TR activity further by increasing the concentration of G418 in the medium led to decreased rates of cell division in trans-dominant recombinant cells but not to lower levels of TR specific activity. Although this may be a reflection of the limits of mutant protein overexpression in the system caused by the asymptotic nature of the response (Fig. 1B), it is also possible that a threshold minimum amount of TR activity might have been reached beyond which parasite growth is no longer sustainable.
T(SH)2 and TR have long been implicated in the protection of trypanosomatid protozoa against oxidative stress (21), but our understanding of the exact mechanism(s) and enzymatic components involved is not yet complete. Recently a T(SH)2-dependant, broad-range hydroperoxide detoxification system was described in Crithidia fasciculata (22) which involves TR and two other enzymatic components: Cf16, a unique oxidoreductase containing a thioredoxin-like motif, and Cf21, a member of the peroxiredoxin family of proteins. In Leishmania, however, iron-superoxide dismutase remains the only well-documented anti-oxidant enzyme (23, 24). Putative nonenzymatic oxidant scavenging mechanisms that have been proposed to operate in this organism include unsaturated lipids (25), surface lypophosphoglycans (26), and the thiol pool (3). Although TR does not participate directly in the removal of reactive oxygen and nitrogen intermediates, it does play a central role in oxidant detoxification through the enzymatic regeneration of the thiol pool. An impaired thiol regeneration ability in cells that express the trans-dominant mutant version of TR is clearly detrimental to parasite intracellular survival (Fig. 5). This finding suggests either the existence of an operational nonenzymatic oxidant scavenging mechanism whose regeneration is fully dependent on T(SH)2 or a dysfunctional T(SH)2-dependent enzymatic detoxification system caused by a rate-limiting provision of cofactor. The later could be analogous to the hydroperoxide detoxification cascade found in C. fasciculata (22).
Direct testing of the involvement of TR in the removal of H2O2 from the parasite was not feasible in our experiments, as this would have required permissive physiological conditions allowing TR to be made rate-limiting. Such conditions appear to be incompatible with life as suggested by our lack of success in further decreasing TR-specific activity in recombinant cells expressing the trans-dominant TR mutant and by the apparent impossibility of creating TR null mutants devoid of catalytic activity by gene targeting (ref. 6; J.T. and A.H.F., unpublished data). Only under extreme oxidative conditions were we able to make TR the rate-limiting enzyme in vitro. Oxidation with diamide resulted in significant differences in the rate of thiol regeneration between wild-type and recombinant cells with decreased or elevated TR activity levels; the direct correlation between TR catalytic activity and thiol regeneration rates observed in our experiments strongly supports the contention that TR is the main thiol-oxidoreductase in this organism.
Infection of macrophages by Leishmania promastigotes entails the initial receptor-mediated recognition of the parasite by the host cell and its active internalization by phagocytosis (reviewed in ref. 27). This process alone triggers the respiratory burst, a host defense response mechanism characterized by the production of reactive oxygen species toxic to the parasite such as superoxide anion (O2−), hydroxyl-radical (⋅OH), and H2O2 (reviewed in ref. 28). Macrophages can be further activated in vitro by exposure to bacterial LPS and IFN-γ, eliciting a further antimicrobial mechanism whose final effector molecule appears to be nitric oxide (NO) and which is dependent on the inducible version of the NO synthase enzyme (iNOS) (ref. 29; reviewed in ref. 30). In our experiments, neither the presence in recombinant Leishmania of plasmid pTEXTcTRtdm nor the expression of trans-dominant mutant protein had a detrimental effect on cell penetration or on parasite survival inside resting macrophages, as indicated respectively by the equivalent number of host cells colonized a few hours after infection and by the equivalent rate of parasite clearance of recombinant and wild-type Leishmania (Fig. 5 A and C). Upon macrophage activation by cytokine however, a dramatic drop in parasite intracellular survival was observed in recombinant Leishmania harboring pTEXTcTRtdm. Because the presence in Leishmania of episomal vectors alone has no detrimental effect on parasite infectivity or viability (6, 31), it is unlikely that the presence of the plasmid per se could be responsible for the increased sensitivity phenotype observed in trans-dominant mutant recombinants. Instead this result suggests an important, nonredundant physiological role for TR during host colonization. Moreover, the ability of recombinant parasites grown in the absence of plasmid selection (with the concomitant recovery of wild-type levels of TR activity) to reverse the observed trans-dominant mutant phenotype (Figs. 5 B and D) is a clear demonstration of the specificity of the mutant phenotype and of the importance of TR for parasite intracellular survival.
In the absence of a cellular oxidative challenge L. donovani grows well even if devoid of up to 85% of its TR catalytic activity. This result would suggest that any rationally designed inhibitor of TR must achieve >85% enzyme inactivation if it is to be considered as a potential anti-leishmanial agent. However, because cells with such diminished TR activity levels are significantly affected in their ability to survive the oxidant challenge mounted by bone marrow macrophages in response to infection, the percentage of TR inhibition of candidate compounds may not need be as high in vivo to be of clinical use. This view is supported by the observation that partial tryA deletion mutants retaining as much as 50% of wild-type TR activity levels also show augmented susceptibility to parasite clearance by macrophages (ref. 6; J.T. and A.H.F., unpublished data). Because Leishmania appears unable to modulate the expression of functional TR homodimers in response to protein inactivation (this study), gene disruption (6), or gene replacement (J.T. and A.H.F., unpublished data), even compounds with moderate but specific TR inhibitory properties are likely to be useful agents for the chemotherapy of leishmanial infections.
Acknowledgments
This work was supported by the Wellcome Trust.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: TR, trypanothione reductase; tryA, trypanothione reductase-encoding gene; ALAT, alanine aminotransferase; T(S)2, trypanothione disulfide; T(SH)2, dihydrotrypanothione; H2O2, hydrogen peroxide; IFN-γ, interferon-γ; LPS, lipopolysaccharide.
References
- 1.Peters W. In: Immunology and Molecular Biology of Parasitic Infections. Warren K S, editor. Oxford: Blackwell Scientific; 1993. pp. 529–566. [Google Scholar]
- 2.Gustafsson L L, Beerman B, Abdi Y A. Handbook of Drugs for Tropical Parasitic Diseases. London: Taylor & Francis; 1987. [Google Scholar]
- 3.Fairlamb A H, Cerami A. Annu Rev Microbiol. 1992;46:695–729. doi: 10.1146/annurev.mi.46.100192.003403. [DOI] [PubMed] [Google Scholar]
- 4.Kelly J M, Taylor M C, Smith K, Hunter K J, Fairlamb A H. Eur J Biochem. 1993;218:29–37. doi: 10.1111/j.1432-1033.1993.tb18348.x. [DOI] [PubMed] [Google Scholar]
- 5.Tovar J, Fairlamb A H. Nucleic Acids Res. 1986;24:2942–2949. doi: 10.1093/nar/24.15.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dumas C, Ouellette M, Tovar J, Cunningham M L, Fairlamb A H, Tamar S, Olivier M, Papadopoulou B. EMBO J. 1997;16:2590–2598. doi: 10.1093/emboj/16.10.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray H W. J Exp Med. 1981;153:1302–1315. doi: 10.1084/jem.153.5.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reiner N E, Kazura J W. Infect Immun. 1982;36:1023–1027. doi: 10.1128/iai.36.3.1023-1027.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borges A, Cunningham M L, Tovar J, Fairlamb A H. Eur J Biochem. 1995;228:745–752. doi: 10.1111/j.1432-1033.1995.tb20319.x. [DOI] [PubMed] [Google Scholar]
- 10.Kelly J M, Ward H W, Miles M A, Kendall G. Nucleic Acids Res. 1992;15:3963–3969. doi: 10.1093/nar/20.15.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 12.Cunningham M L, Zvelebil J J M, Fairlamb A H. Eur J Biochem. 1994;221:285–295. doi: 10.1111/j.1432-1033.1994.tb18740.x. [DOI] [PubMed] [Google Scholar]
- 13.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 14.Taylor M C, Kelly J M, Chapman C J, Fairlamb A H, Miles M A. Mol Biochem Parasitol. 1994;64:293–301. doi: 10.1016/0166-6851(94)00034-4. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham M L, Fairlamb A H. Eur J Biochem. 1995;230:460–468. doi: 10.1111/j.1432-1033.1995.tb20583.x. [DOI] [PubMed] [Google Scholar]
- 16.Medina Acosta E, Cross G A M. Mol Biochem Parasitol. 1993;59:327–329. doi: 10.1016/0166-6851(93)90231-l. [DOI] [PubMed] [Google Scholar]
- 17.Feinberg A P, Vogelstein B. Anal Biochem. 1984;137:266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- 18.Shim H, Fairlamb A H. J Gen Microbiol. 1988;134:807–817. doi: 10.1099/00221287-134-3-807. [DOI] [PubMed] [Google Scholar]
- 19.Penketh P G, Klein R A. Mol Biochem Parasitol. 1986;20:111–121. doi: 10.1016/0166-6851(86)90023-x. [DOI] [PubMed] [Google Scholar]
- 20.Kiderlen A F, Kaye P M. J Immunol Methods. 1990;127:11–18. doi: 10.1016/0022-1759(90)90334-r. [DOI] [PubMed] [Google Scholar]
- 21.Henderson G B, Fairlamb A H, Cerami A. Mol Biochem Parasitol. 1987;24:39–45. doi: 10.1016/0166-6851(87)90113-7. [DOI] [PubMed] [Google Scholar]
- 22.Nogoceke E, Gommel D U, Kiess M, Kalisz H M, Flohe L. Biol Chem. 1987;378:827–836. doi: 10.1515/bchm.1997.378.8.827. [DOI] [PubMed] [Google Scholar]
- 23.LeTrang N, Meshnick S R, Kitchener K, Eaton J W, Cerami A. J Biol Chem. 1983;258:125–130. [PubMed] [Google Scholar]
- 24.Ismail S O, Skeiky Y A W, Bhatia A, Omara-Opyene L A, Gedamu L. Infect Immun. 1994;62:657–664. doi: 10.1128/iai.62.2.657-664.1994. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Jackett P S, Aber V R, Lowrie D B. J Gen Microbiol. 1978;104:37–45. doi: 10.1099/00221287-104-1-37. [DOI] [PubMed] [Google Scholar]
- 26.Chan J, Fujiwara T, Brennan P, McNeil M, Turco S J, Sibille J C, Snapper M, Aisen P, Bloom B R. Proc Natl Acad Sci USA. 1989;86:2453–2457. doi: 10.1073/pnas.86.7.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sacks D L, Louis J A, Wirth D F. In: Immunology and Molecular Biology of Parasitic Infections. Warren K S, editor. Oxford: Blackwell Scientific; 1993. pp. 237–268. [Google Scholar]
- 28.Rosen G M, Pou S, Ramos C L, Cohen M S, Britigan B E. FASEB J. 1995;9:200–209. doi: 10.1096/fasebj.9.2.7540156. [DOI] [PubMed] [Google Scholar]
- 29.Barton C H, Whitehead S H, Blackwell J M. Mol Med. 1995;1:267–279. [PMC free article] [PubMed] [Google Scholar]
- 30.James S L. Microbiol Rev. 1995;59:533–547. doi: 10.1128/mr.59.4.533-547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mottram J C, Souza A E, Hutchison J E, Carter R, Frame M J, Coombs G H. Proc Natl Acad Sci USA. 1996;93:6008–6013. doi: 10.1073/pnas.93.12.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]