Abstract
A Mycobacterium sp., designated strain BG1, able to utilize the polycyclic aromatic hydrocarbon phenanthrene as the sole carbon and energy source was isolated from estuarine sediment following enrichment with the hydrocarbon. Unlike other phenanthrene degraders, this bacterium degraded phenanthrene via 1-hydroxy-2-naphthoic acid without accumulating this or other aromatic intermediates, as shown by high-performance liquid chromatography. Degradation proceeded via meta cleavage of protocatechuic acid. Different nonionic surfactants (Tween compounds) solubilized the phenanthrene to different degrees and enhanced phenanthrene utilization. The order of enhancement, however, did not correlate perfectly with increased solubility, suggesting physiological as well as physicochemical effects of the surfactants. Plasmids of approximately 21, 58, and 77 megadaltons were detected in cells grown with phenanthrene but not in those which, after growth on nutrient media, lost the phenanthrene-degrading phenotype. Given that plasmid-mediated degradations of aromatic hydrocarbons generally occur via meta cleavages, it is of interest that the addition of pyruvate, a product of meta cleavage, supported rapid mineralization of phenanthrene in broth culture; succinate, a product of ortho cleavage, supported growth but completely repressed the utilization of phenanthrene. The involvement of plasmids may have given rise to the unusual degradation pattern that was observed.
Full text
PDF
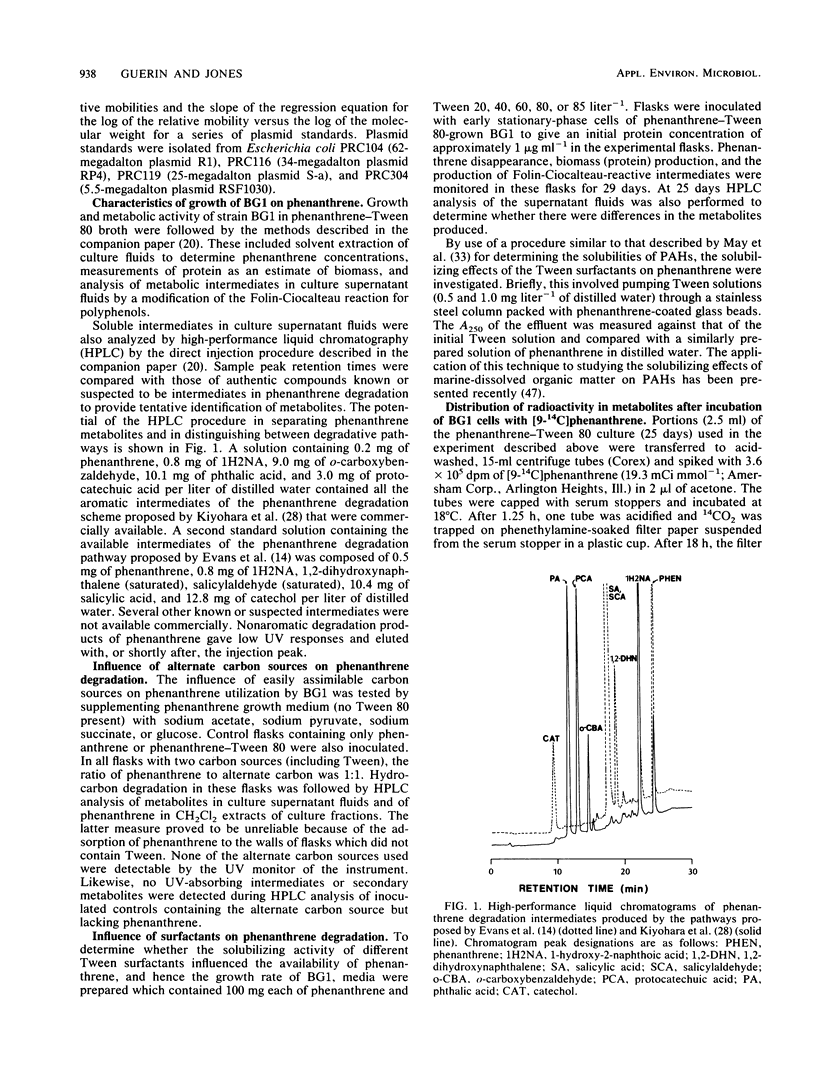
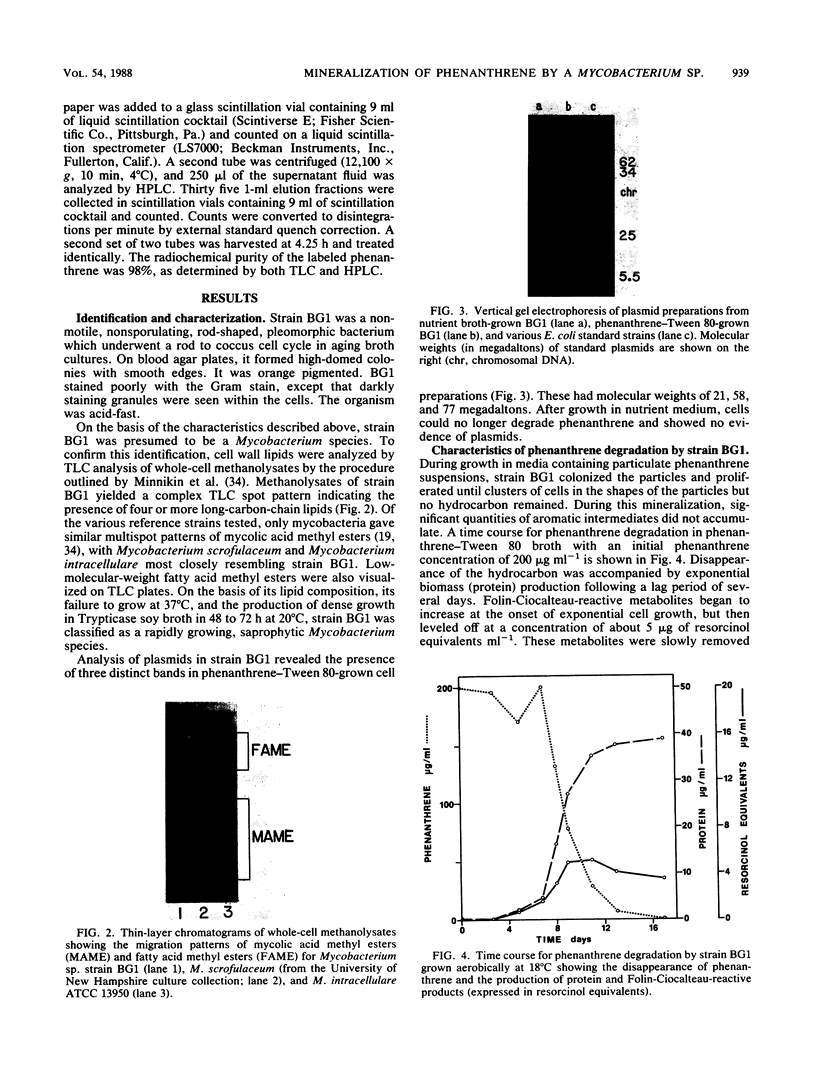


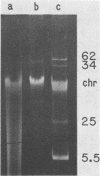
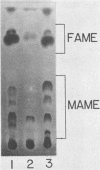
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnsley E. A. Phthalate pathway of phenanthrene metabolism: formation of 2'-carboxybenzalpyruvate. J Bacteriol. 1983 Apr;154(1):113–117. doi: 10.1128/jb.154.1.113-117.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell L. D., Haas H. F. The Utilization of Certain Hydrocarbons by Microorganisms. J Bacteriol. 1941 May;41(5):653–673. doi: 10.1128/jb.41.5.653-673.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerniglia C. E. Microbial metabolism of polycyclic aromatic hydrocarbons. Adv Appl Microbiol. 1984;30:31–71. doi: 10.1016/s0065-2164(08)70052-2. [DOI] [PubMed] [Google Scholar]
- Chakrabarty A. M. Genetic basis of the biodegradation of salicylate in Pseudomonas. J Bacteriol. 1972 Nov;112(2):815–823. doi: 10.1128/jb.112.2.815-823.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty A. M. Plasmids in Pseudomonas. Annu Rev Genet. 1976;10:7–30. doi: 10.1146/annurev.ge.10.120176.000255. [DOI] [PubMed] [Google Scholar]
- Clarke P. H. The metabolic versatility of pseudomonads. Antonie Van Leeuwenhoek. 1982 May;48(2):105–130. doi: 10.1007/BF00405197. [DOI] [PubMed] [Google Scholar]
- Collins C. H., Grange J. M., Yates M. D. Mycobacteria in water. J Appl Bacteriol. 1984 Oct;57(2):193–211. doi: 10.1111/j.1365-2672.1984.tb01384.x. [DOI] [PubMed] [Google Scholar]
- Cook A. M., Grossenbacher H., Hütter R. Isolation and cultivation of microbes with biodegradative potential. Experientia. 1983 Nov 15;39(11):1191–1198. doi: 10.1007/BF01990356. [DOI] [PubMed] [Google Scholar]
- Cox D. P., Williams A. L. Biological Process for Converting Naphthalene to cis-1,2-Dihydroxy-1,2-Dihydronaphthalene. Appl Environ Microbiol. 1980 Feb;39(2):320–326. doi: 10.1128/aem.39.2.320-326.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn N. W., Gunsalus I. C. Transmissible plasmid coding early enzymes of naphthalene oxidation in Pseudomonas putida. J Bacteriol. 1973 Jun;114(3):974–979. doi: 10.1128/jb.114.3.974-979.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS W. C., FERNLEY H. N., GRIFFITHS E. OXIDATIVE METABOLISM OF PHENANTHRENE AND ANTHRACENE BY SOIL PSEUDOMONADS. THE RING-FISSION MECHANISM. Biochem J. 1965 Jun;95:819–831. doi: 10.1042/bj0950819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOSTER J. W. Hydrocarbons as substrates for microorganisms. Antonie Van Leeuwenhoek. 1962;28:241–274. doi: 10.1007/BF02538739. [DOI] [PubMed] [Google Scholar]
- Goodfellow M., Collins M. D., Minnikin D. E. Thin-layer chromatographic analysis of mycolic acid and other long-chain components in whole-organism methanolysates of coryneform and related taxa. J Gen Microbiol. 1976 Oct;96(2):351–358. doi: 10.1099/00221287-96-2-351. [DOI] [PubMed] [Google Scholar]
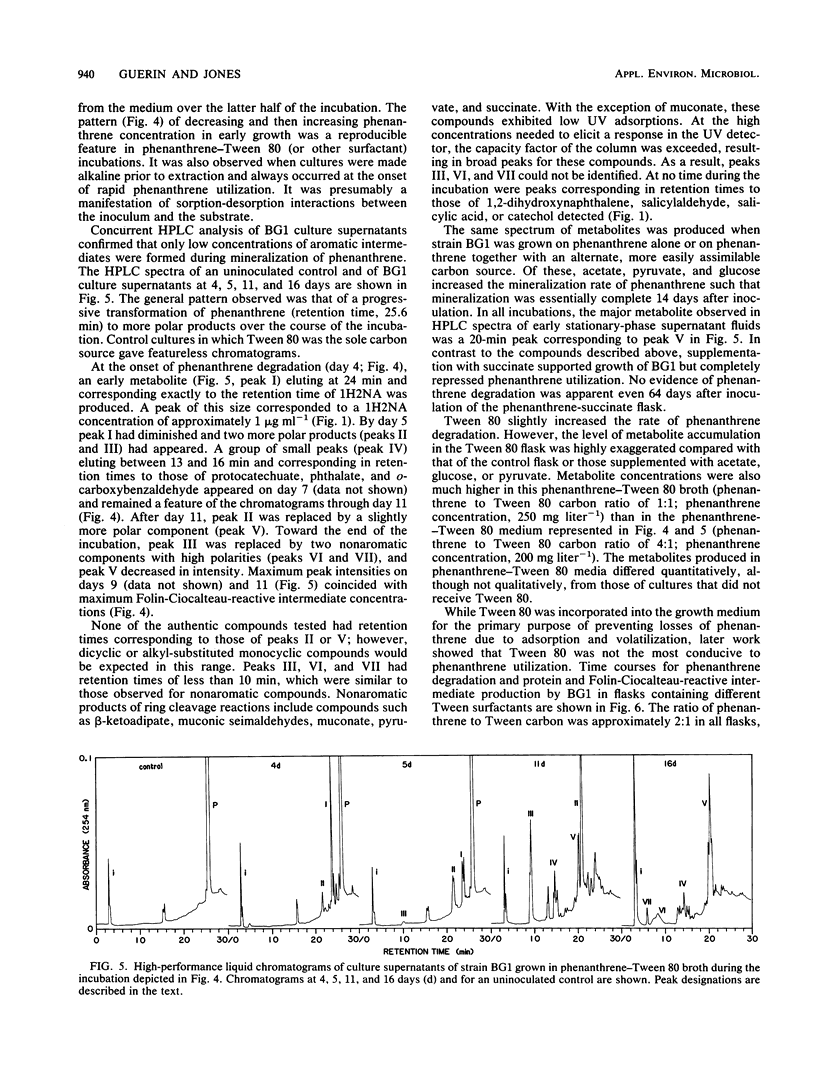
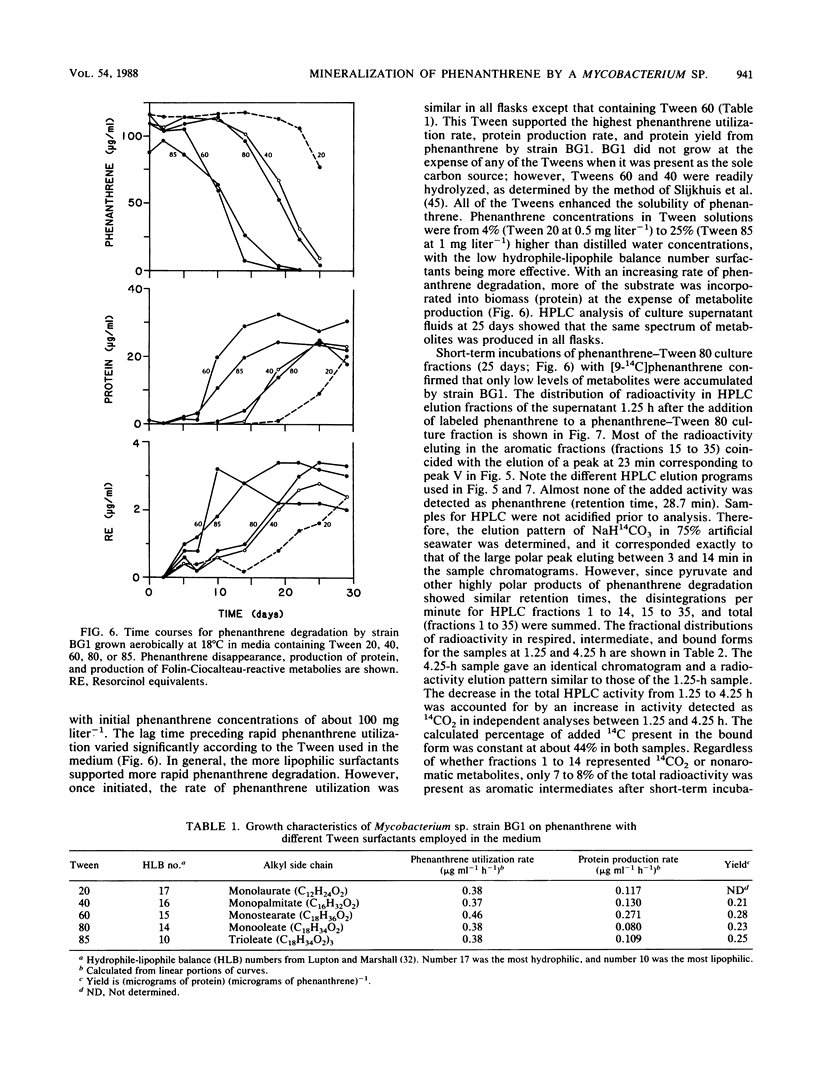
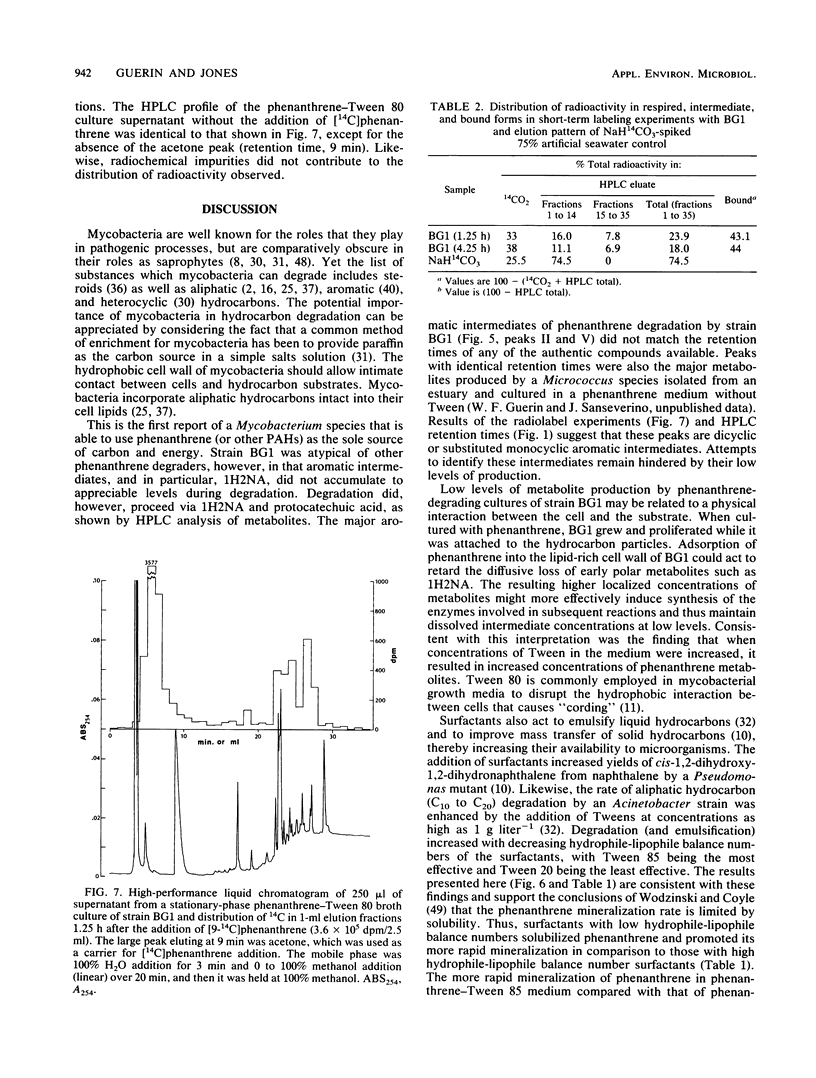
- Guerin W. F., Jones G. E. Two-stage mineralization of phenanthrene by estuarine enrichment cultures. Appl Environ Microbiol. 1988 Apr;54(4):929–936. doi: 10.1128/aem.54.4.929-936.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas D. Genetic aspects of biodegradation by pseudomonads. Experientia. 1983 Nov 15;39(11):1199–1213. doi: 10.1007/BF01990357. [DOI] [PubMed] [Google Scholar]
- Hedgecock L. W. Nutritional characteristics of the atypical mycobacteria. J Bacteriol. 1968 Aug;96(2):306–313. doi: 10.1128/jb.96.2.306-313.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerina D. M., Selander H., Yagi H., Wells M. C., Davey J. F., Mahadevan V., Gibson D. T. Dihydrodiols from anthracene and phenanthrene. J Am Chem Soc. 1976 Sep 15;98(19):5988–5996. doi: 10.1021/ja00435a035. [DOI] [PubMed] [Google Scholar]
- King D. H., Perry J. J. The origin of fatty acids in the hydrocarbon-utilizing microorganism Mycobacterium vaccae. Can J Microbiol. 1975 Jan;21(1):85–89. doi: 10.1139/m75-012. [DOI] [PubMed] [Google Scholar]
- Kiyohara H., Nagao K., Kouno K., Yano K. Phenanthrene-degrading phenotype of Alcaligenes faecalis AFK2. Appl Environ Microbiol. 1982 Feb;43(2):458–461. doi: 10.1128/aem.43.2.458-461.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyohara H., Sugiyama M., Mondello F. J., Gibson D. T., Yano K. Plasmid involvement in the degradation of polycyclic aromatic hydrocarbons by a Beijerinckia species. Biochem Biophys Res Commun. 1983 Mar 29;111(3):939–945. doi: 10.1016/0006-291x(83)91390-6. [DOI] [PubMed] [Google Scholar]
- Krulwich T. A., Pelliccione N. J. Catabolic pathways of coryneforms, nocardias, and mycobacteria. Annu Rev Microbiol. 1979;33:95–111. doi: 10.1146/annurev.mi.33.100179.000523. [DOI] [PubMed] [Google Scholar]
- Minnikin D. E., Alshamaony L., Goodfellow M. Differentiation of Mycobacterium, Nocardia, and related taxa by thin-layer chromatographic analysis of whole-organism methanolysates. J Gen Microbiol. 1975 May;88(1):200–204. doi: 10.1099/00221287-88-1-200. [DOI] [PubMed] [Google Scholar]
- Monticello D. J., Bakker D., Finnerty W. R. Plasmid-mediated degradation of dibenzothiophene by Pseudomonas species. Appl Environ Microbiol. 1985 Apr;49(4):756–760. doi: 10.1128/aem.49.4.756-760.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G. L., Perry J. J. Incorporation of chlorinated alkanes into fatty acids of hydrocarbon-utilizing mycobacteria. J Bacteriol. 1983 Dec;156(3):1158–1164. doi: 10.1128/jb.156.3.1158-1164.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGOFF M. H., WENDER I. The microbiology of coal. I. Bacterial oxidation of phenanthrene. J Bacteriol. 1957 Feb;73(2):264–268. doi: 10.1128/jb.73.2.264-268.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer W. B., Lewis C. W., Jr Effect of oleic acid on growth and cell structure of mycobacteria. J Bacteriol. 1965 Nov;90(5):1438–1447. doi: 10.1128/jb.90.5.1438-1447.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skriabin G. K., Starovoitov I. I., Borisoglebskaia A. N., Borodin A. M. Okislenie naftalina shtammom Pseduomonas putida, nesushchim mutantnuiu plazmidu. Mikrobiologiia. 1978 Mar-Apr;47(2):273–277. [PubMed] [Google Scholar]
- Slijkhuis H., van Groenestijn J. W., Kylstra D. J. Microthrix parvicella, a filamentous bacterium from activated sludge: growth on Tween 80 as carbon and energy source. J Gen Microbiol. 1984 Aug;130(8):2035–2042. doi: 10.1099/00221287-130-8-2035. [DOI] [PubMed] [Google Scholar]
- West P. A., Okpokwasili G. C., Brayton P. R., Grimes D. J., Colwell R. R. Numerical taxonomy of phenanthrene-degrading bacteria isolated from the Chesapeake Bay. Appl Environ Microbiol. 1984 Nov;48(5):988–993. doi: 10.1128/aem.48.5.988-993.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodzinski R. S., Coyle J. E. Physical state of phenanthrene for utilization by bacteria. Appl Microbiol. 1974 Jun;27(6):1081–1084. doi: 10.1128/am.27.6.1081-1084.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodzinski R. S., Johnson M. J. Yields of bacterial cells from hydrocarbons. Appl Microbiol. 1968 Dec;16(12):1886–1891. doi: 10.1128/am.16.12.1886-1891.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuniga M. C., Durham D. R., Welch R. A. Plasmid- and chromosome-mediated dissimilation of naphthalene and salicylate in Pseudomonas putida PMD-1. J Bacteriol. 1981 Sep;147(3):836–843. doi: 10.1128/jb.147.3.836-843.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]