Abstract
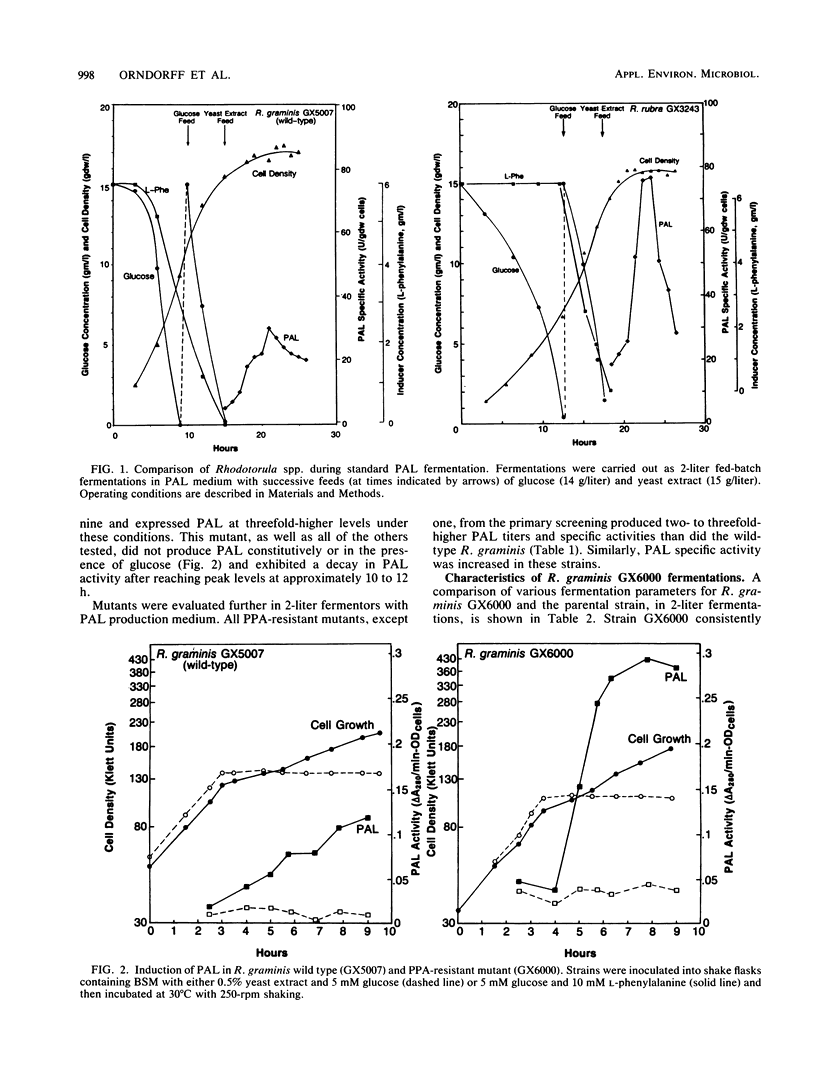
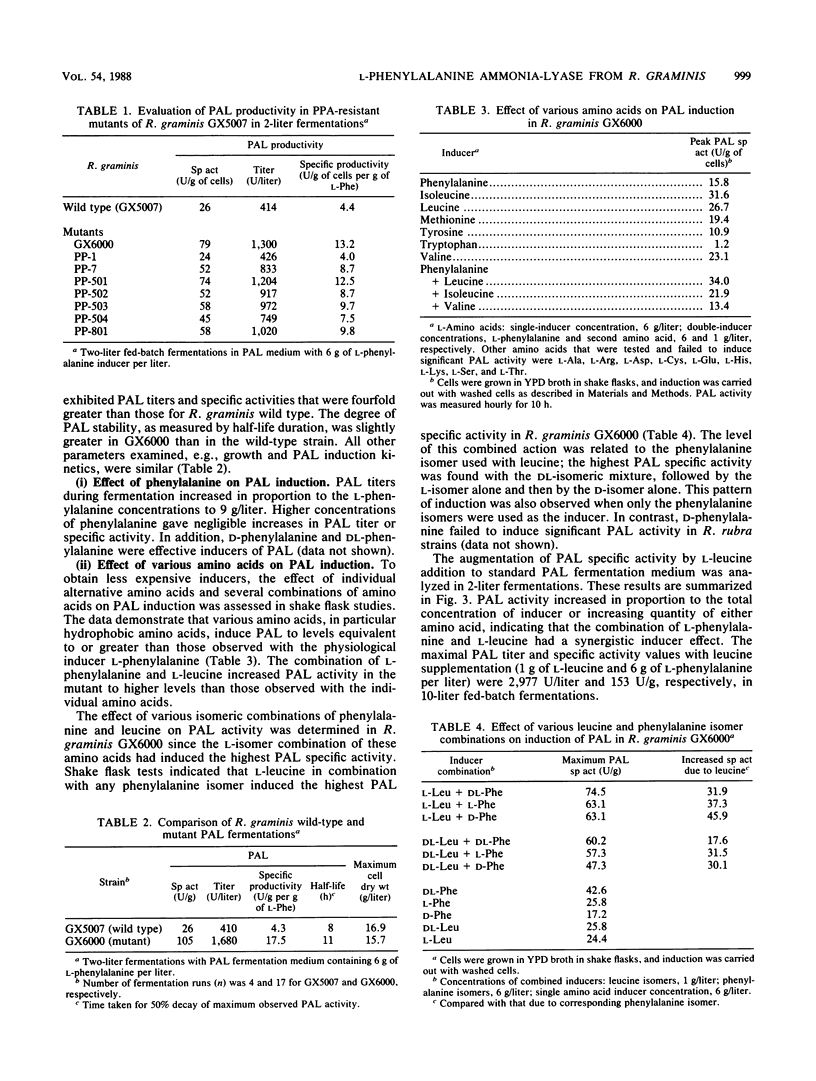
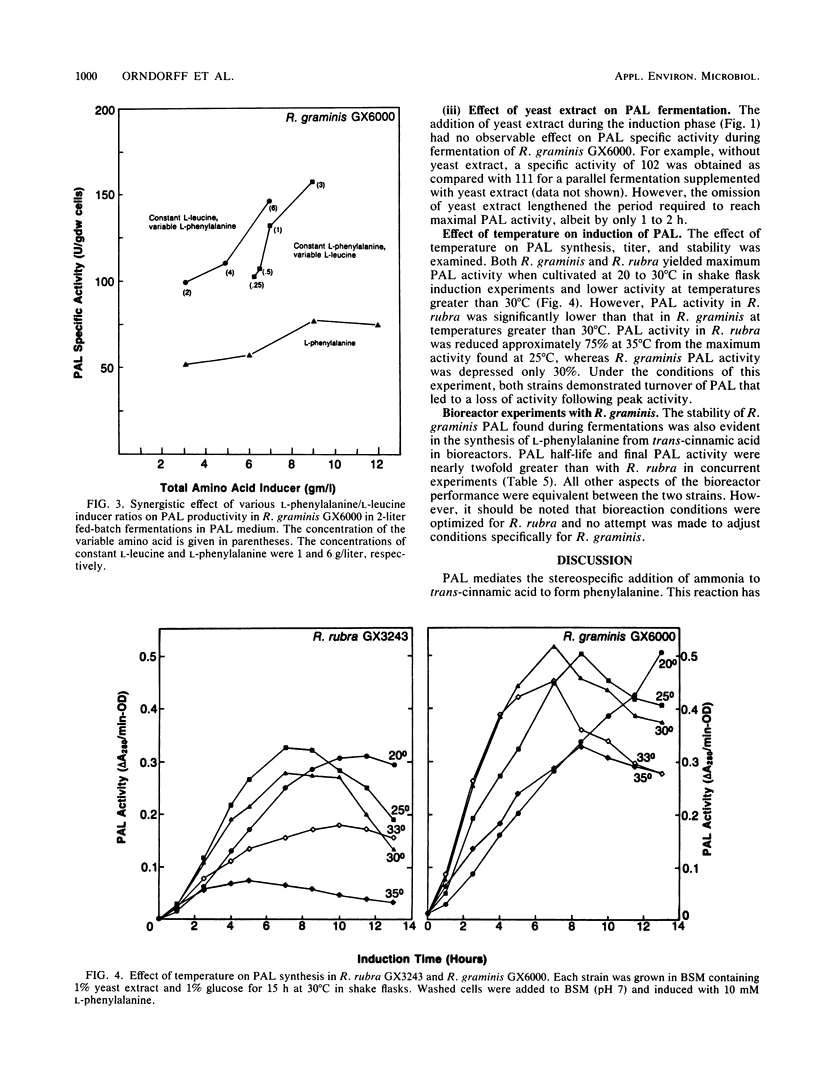
l-Phenylalanine ammonia-lyase (PAL; EC 4.3.1.5) from Rhodotorula rubra has been used in the commercial manufacture of l-phenylalanine from trans-cinnamic acid. In this study, R. graminis PAL was investigated. Mutant strain GX6000 was isolated after ethyl methanesulfonate mutagenesis of wild-type R. graminis GX5007 by selecting for resistance to phenylpropiolic acid, an analog of trans-cinnamic acid. Mutant strain GX6000 produced inducible PAL at levels four- to fivefold higher than had wild-type R. graminis. Furthermore, this strain had several other physiological traits that make it more commercially useful than R. rubra. For example, during fermentation, the PAL half-life was three- to fivefold longer, PAL specific activity was six to seven times higher, and PAL synthesis was significantly less inhibited by temperatures above 30°C. Induction of PAL in strain GX6000 appeared to be less tightly regulated; l-leucine acted synergistically with l-phenylalanine, the physiological inducer, to increase the PAL specific activity and titer to 165 U/g (dry weight) and 3,000 U/liter, respectively, a 40% increase over the effect of l-phenylalanine alone. Strain GX6000 PAL showed significantly greater stability in bioreactors for the synthesis of l-phenylalanine, a finding that is consistent with the stability properties observed during fermentation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Durham D. R., McNamee C. G., Stewart D. B. Dissimilation of aromatic compounds in Rhodotorula graminis: biochemical characterization of pleiotropically negative mutants. J Bacteriol. 1984 Nov;160(2):771–777. doi: 10.1128/jb.160.2.771-777.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham D. R., Phibbs P. V., Jr Fractionation and characterization of the phosphoenolpyruvate: fructose 1-phosphotransferase system from Pseudomonas aeruginosa. J Bacteriol. 1982 Feb;149(2):534–541. doi: 10.1128/jb.149.2.534-541.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz R. R., Hodgins D. S., Abell C. W. Phenylalanine ammonia-lyase. Induction and purification from yeast and clearance in mammals. J Biol Chem. 1976 Aug 10;251(15):4646–4650. [PubMed] [Google Scholar]
- Gilbert H. J., Tully M. Synthesis and degradation of phenylalanine ammonia-lyase of Rhodosporidium toruloides. J Bacteriol. 1982 May;150(2):498–505. doi: 10.1128/jb.150.2.498-505.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegeman G. D., Root R. T. The effect of a non-metabolizable analog on mandelate catabolism in Pseudomonas putida. Arch Microbiol. 1976 Oct 11;110(1):19–25. doi: 10.1007/BF00416964. [DOI] [PubMed] [Google Scholar]
- Hodgins D. S. Yeast phenylalanine ammonia-lyase. Purification, properties, and the identification of catalytically essential dehydroalanine. J Biol Chem. 1971 May 10;246(9):2977–2985. [PubMed] [Google Scholar]
- Hoskins J. A., Jack G., Wade H. E., Peiris R. J., Wright E. C., Starr D. J., Stern J. Enzymatic control of phenylalanine intake in phenylketonuria. Lancet. 1980 Feb 23;1(8165):392–394. doi: 10.1016/s0140-6736(80)90944-7. [DOI] [PubMed] [Google Scholar]
- Kalghatgi K. K., Subba Rao P. V. Microbial L-phenylalanine ammonia-lyase. Purification, subunit structure and kinetic properties of the enzyme from Rhizoctonia solani. Biochem J. 1975 Jul;149(1):65–72. doi: 10.1042/bj1490065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore G., Sugumaran M., Vaidyanathan C. S. Metabolism of DL-(+/-)-phenylalanine by Aspergillus niger. J Bacteriol. 1976 Oct;128(1):182–191. doi: 10.1128/jb.128.1.182-191.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama H. A simple and rapid enzymatic determination of L-phenylalanine with a novel L-phenylalanine oxidase (deaminating and decarboxylating) from Pseudomonas sp. P-501. Clin Chim Acta. 1984 Jan 31;136(2-3):131–136. doi: 10.1016/0009-8981(84)90284-5. [DOI] [PubMed] [Google Scholar]
- Marusich W. C., Jensen R. A., Zamir L. O. Induction of L-phenylalanine ammonia-lyase during utilization of phenylalanine as a carbon or nitrogen source in Rhodotorula glutinis. J Bacteriol. 1981 Jun;146(3):1013–1019. doi: 10.1128/jb.146.3.1013-1019.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misawa T., Aikawa H., Shigeta S. [Effects of alcohol drinking on mental task performance]. Sangyo Igaku. 1983 Sep;25(5):406–414. doi: 10.1539/joh1959.25.406. [DOI] [PubMed] [Google Scholar]
- Wick J. F., Willis J. E. Phenylalanine-dependent de novo synthesis of phenylalanine ammonia-lyase from Rhodotorula glutinis. Arch Biochem Biophys. 1982 Jul;216(2):385–391. doi: 10.1016/0003-9861(82)90226-0. [DOI] [PubMed] [Google Scholar]
- Yamada S., Nabe K., Izuo N., Nakamichi K., Chibata I. Production of l-Phenylalanine from trans-Cinnamic Acid with Rhodotorula glutinis Containing l-Phenylalanine Ammonia-Lyase Activity. Appl Environ Microbiol. 1981 Nov;42(5):773–778. doi: 10.1128/aem.42.5.773-778.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


