Abstract
The cerebral cortex is parcellated into different functional domains that receive distinct inputs from other cortical and subcortical regions. The molecular mechanisms underlying the specificity of connections of cortical afferents remain unclear. We report here that the Eph family tyrosine kinase receptor EphA5 and the ligand ephrin-A5 may play a key role in the exclusion of the limbic thalamic afferents from the sensorimotor cortex by mediating repulsive interactions. In situ hybridization shows that the EphA5 transcript is expressed at high levels in both cortical and subcortical limbic regions, including the frontal cortex, the subiculum, and the medial thalamic nuclei. In contrast, ephrin-A5 is transcribed abundantly in the sensorimotor cortex. Consistent with the complementary expression, the ligand inhibited dramatically the growth of neurites from neurons isolated from the medial thalamus but was permissive for the growth of neurites from lateral thalamic neurons, which is primarily nonlimbic. Similarly, the growth of neurites from Eph-A5-expressing neurons isolated from the subiculum was inhibited by ephrin-A5. Our studies suggest that the Eph family ligand ephrin-A5 serves as a general inhibitor of axonal growth from limbic neurons, which may serve to prevent innervation of inappropriate primary sensorimotor regions, thus contributing to the generation of specificity of thalamic cortical afferents.
The cerebral cortex is parcellated into multiple domains that subserve different functions (1–3). The medial cortical areas, which include prefrontal, cingulate, and retrosplenial cortices, are components of limbic circuits, in contrast to nonlimbic sensory and motor cortices (3, 4). The limbic cortices receive projections from nuclei in the medial and anterior thalamus and from other limbic areas, including the hippocampal formation (5). In contrast, the sensorimotor cortex is innervated specifically by primary sensory and motor thalamic nuclei located in the ventrolateral and posterior thalamus (5). This topographic arrangement of mature functional pathways in the adult brain is paralleled by an early specificity of thalamocortical projections during development (2, 6, 7). This specificity suggests the presence of guidance mechanisms that facilitate the formation of distinct projection patterns.
In the classic model of topographic map formation, Sperry (8, 9) proposed that molecular tags form gradients in projecting and target fields and interact to guide axons to appropriate regions. Such guidance cues have been identified recently and have been shown to be receptors and ligands of the Eph family of tyrosine kinases (10–13). In the retinotectal topographic map, the Eph family receptor EphA3 is expressed by retinal ganglion neurons in a nasal (low) to temporal (high) gradient whereas two ligands, ephrin-A2 and -A5, are distributed in a complementary anterior (low) to posterior (high) gradient in the tectum (10, 14). In the hippocamposeptal projection, the Eph family receptor EphA5 is expressed in a lateral (low) to medial (high) gradient in the hippocampus, and at least three ligands, ephrin-A2, -A3, and -A5, form an opposing gradient in the septal target (11, 15). It has been proposed that multiple Eph ligands may function in combination spatially to specify the target field (12, 13, 15). Consistent with the opposing gradients, the interaction between the receptors and the ligands has been shown to generate inhibitory/repulsive activity on the growth of axons, thus invoking a mechanism of axon guidance that relies in part on negative cues to prevent ingrowth into inappropriate targets (10–12).
In the present report, we examine the role of Eph family receptors and ligands in the guidance of thalamocortical afferents to their correct target area. We demonstrate that the Eph ligand ephrin-A5 is expressed densely in the sensorimotor cortex but not in the limbic cortex. Complementary to the ligand expression, we show that the Eph family receptor EphA5 is expressed primarily in medial (limbic) nuclei but not in the lateral and ventral (sensorimotor) nuclei of the thalamus. Furthermore, we show that ephrin-A5 reduces the length of thalamic and cortical limbic axons but has no effect on thalamic sensorimotor axonal outgrowth. Our observations suggest that ephrin-A5 may serve as a repulsive guidance cue to prevent limbic axons from invading the somatosensory cortex.
MATERIALS AND METHODS
In Situ Hybridization.
Localization of the transcripts encoding the Eph family receptor EphA5 and its ligand ephrin-A5 was performed by using methods described (16). EphA5 transcripts were detected with a 373-bp riboprobe from the extracellular and transmembrane domain of EphA5 (16). Ephrin-A5 expression was detected with an anti-sense riboprobe transcribed from a 0.7-kb human AL-1/LERK7 cDNA cloned in pBluescript (Stratagene) and containing the entire coding region of the ligand (17). Human and murine ephrin-A5 share >90% homology at the nucleotide level. The probe detects murine Lerk7 specifically and does not hybridize with other Eph family ligands, as demonstrated by Southern blot analysis of murine genomic DNA (D.P.C., unpublished data). A sense probe was used as a control, and it revealed no specific binding.
Expression of Recombinant Ephrin-A5.
Human ephrin-A5 was expressed by using a retroviral vector, pLIG*, that contains a β-galactosidase gene fused to an aminoglycoside phosphotransferase for G418 selection (18) as reported (11). The expression construct was transfected into NIH 3T3 cells. G418-resistant colonies then were screened for ephrin-A5 expression by using the extracellular domain of the Eph receptor EphA5 fused to alkaline phosphatase (11). The expression was confirmed further by Northern blot analysis (data not shown). Ephrin-A5-transfected cell lines expressing high levels of EphA5–alkaline phophatase binding activity were used in the in vitro assays.
Neurite Outgrowth Assay.
The effect of ephrin-A5 on the growth of neurites was assayed according to Gao et al. (11) with some modifications. In brief, the dorsal thalamus from an embryonic day 18 rat was dissected by using the external medullary lamina and the medial ventricular sulcus of His as ventral landmarks. Each piece was bisected in the dorsoventral direction. The medial aspect contains the mediodorsal and centromedial nuclei, which project to perirhinal and frontal cortical regions (4, 5). We refer to these thalamic regions as “limbic” for simplicity. The lateral aspect contains the ventrobasal complex, the ventral motor region, and the dorsal lateral geniculate nuclei, which project to primary sensory and motor cortical areas, and the posterior nucleus, which projects diffusely throughout limbic and sensory cortices (4, 5). In some experiments, the subicular complex, situated lateral to the hippocampus proper, was dissected and assayed for neurite outgrowth.
Dissected tissues were dissociated with trituration to a single cell suspension and plated at a density of 5 × 104 cells/well in 12-well dishes previously seeded with a confluent monolayer of ephrin-A5-expressing or control NIH 3T3 cells transfected with the vector. Cells were grown in DMEM supplemented with fetal bovine serum (10%), penicillin (0.5 unit/ml), and streptomycin (0.5 μg/ml) for 48 hr, fixed in 4% paraformaldehyde in PBS, and stained with anti-neuron-specific enolase with a Vectastain ABC kit (Vector Laboratories). The lengths of neurites were measured under a Zeiss Telaval 31 microscope in 10 randomly selected fields. All neurons in selected fields were included in the quantitation. Experiments were repeated at least three times with three duplicate dishes in each experiment. Two measures were tabulated: mean neurite length and the population distribution of neurons whose longest neurite was longer than a specific length (19, 20).
RESULTS
Expression of the Eph Family Receptor EphA5 in the Thalamus.
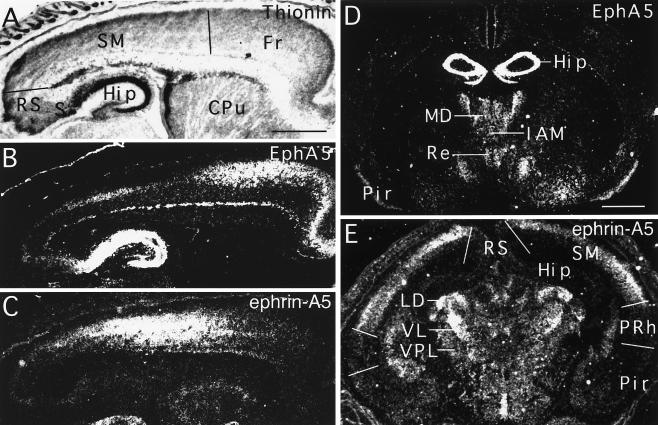
We have shown previously that EphA5 is expressed at high levels in the neural structures of the limbic system in general and colocalizes with the transcript encoding the limbic system-associated membrane protein in the adult brain (16, 21). Here, we examined in more detail the expression of EphA5 in the thalamus by using in situ hybridization as described (16). EphA5 is transcribed at high levels in the midline thalamic nuclei, including the anterior dorsal, mediodorsal, central medial, interanteriomedial, paraventricular, intermediodorsal, rhomboid, and reuniens nuclei (Fig. 1 and data not shown). The specific expression in the medial thalamus was detected as early as embryonic day 16 and persisted in the adult at low levels. The most dense labeling was detected during late embryogenesis and during the early postnatal period (embryonic day 16 until postnatal day 3), when the peak of initial establishment of thalamocortical projections occurs (6, 22–26). In contrast, low EphA5 expression was detected in the principle relay nuclei, such as the medial and dorsal lateral geniculate nuclei, ventral medial and lateral nuclei, ventral posterior nucleus, and posterior nucleus (Fig. 1 and data not shown). These data revealed an abundant expression of EphA5 in the thalamic association nuclei but not in the principle sensory and motor relay nuclei and are consistent with a role for this Eph receptor in the establishment of area-specific thalamocortical projections.
Figure 1.
Complimentary expression of the Eph family receptor EphA5 and ligand ephrin-A5 in the cerebral cortex and thalamus. (A and B) Bright- and dark-field photomicrographs, respectively, of parasagittal P3 murine brain section hybridized with anti-sense EphA5 probe. High levels of receptor expression are limited to limbic cortices such as the frontal cortex and the hippocampus formation. (C) Dark-field photomicrograph of a serial section to that shown in A and B, hybridized to the anti-sense probe of ephrin-A5. (D) Dark-field photomicrograph of a coronal section of P7 murine brain hybridized with anti-sense EphA5 probe, showing that the receptor is expressed at high levels only in the medial thalamus. (E) Dark-field photomicrograph of a coronal section of P3 murine brain hybridized with anti-sense ephrin-A5 probe. Ephrin-A5 was detected primarily in the sensorimotor cortex and certain lateral thalamic nuclei. The approximate boundaries between different cortical areas are marked by thin lines. (Cpu, caudate-putamen; Fr, frontal cortex; Hip, hippocampus, IAM, interanterodorsal nucleus; LD, lateral dorsal nucleus; MD, medial dorsal nucleus; Pir, piriform cortex; PRh, perirhinal cortex; Re, nucleus reunions; RS, retrosplenial cortex; S, subiculum; SM, sensorimotor cortex; VL, ventral lateral nucleus; VPL, ventral posterior lateral nucleus.) [Bars = 0.63 mm (A), = 1 mm (D).]
Expression of Ephrin-A5 in the Cortex.
The EphA5-positive thalamic nuclei form specific projections to the limbic cortex but not to the sensorimotor cortex. To establish the potential for a specific interaction between EphA5 and its ligand(s) in the regulation of limbic thalamic targeting, we investigated ligand expression patterns throughout the developing and adult cerebral cortex. Ephrin-A5 transcripts are expressed widely throughout the sensorimotor and auditory cortices (Fig. 1), decreasing to very low levels just dorsal to the rhinal fissure (Fig. 1). Moderate levels of mRNA transcripts also were detected in primary visual cortex (data not shown). In contrast, only very low levels of expression were observed in the superficial layers of the cingulate cortex, and no detectable expression was found in the retrosplenial cortex, the piriform cortex, or the hippocampal formation (Fig. 1). Expression of ephrin-A5 transcript was evident first at low but detectable levels in the sensorimotor cortex at E16 in the cortical plate and increased to peak intensity during late embryogenesis and the early postnatal period (Fig. 1 and data not shown). These results indicate that ephrin-A5 expression is restricted to primary sensory and motor regions of neocortex, with a general lack of expression in allocortical and mesocortical limbic regions. The complementary expression patterns of EphA5 and ephrin-A5 are consistent with a hypothesis that interaction between ephrin-A5 and EphA5 may prevent innervation of the sensory and motor neocortices by neurons in limbic thalamic nuclei.
Selective Inhibition of Medial Thalamic Neurite Outgrowth by Ephrin-A5.
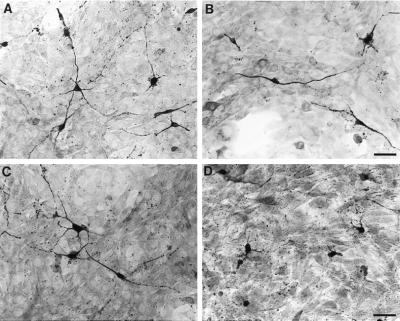
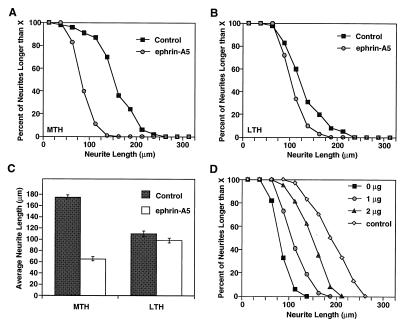
A critical prediction of our hypothesis that ephrin-A5 might act to restrict limbic projections is that axonal growth from the medial thalamus containing limbic cortex-projecting neurons but not from the lateral thalamus should be inhibited in the presence of the Eph family ligand. To examine the effect of ephrin-A5 on neurite growth of medial and lateral thalamic neurons, we constructed NIH 3T3 cell lines expressing high levels of ephrin-A5 as described (11). Neurons from the medial or lateral thalamus were dissected and plated on a monolayer of control or ephrin-A5-expressing 3T3 cells. Both the medial and the lateral neurons grew extensive neurites on the control cells (Fig. 2 A and B). In contrast, when cocultured with the ligand-expressing cells, only the lateral thalamic neurons grew long neurites on ephrin-A5-expressing cells (Fig. 2 C and D). The medial neurons exhibited significantly shorter neurites compared with their growth on the same cellular substrate in the absence of ephrin-A5 (Figs. 2 B and D and 3A). Analysis of the population distribution of the length of the neurites showed that, in control cells, >90% of medial neurons had neurites longer than 100 μm whereas on ephrin-A5-expressing cells, only ≈30% of neurons had neurites longer than 100 μm (Fig. 3A). Comparison of the average neurite length showed that there is a 63% decrease in neurite outgrowth by medial neurons exposed to ephrin-A5 (Fig. 3C). No significant inhibition was observed for the growth of the lateral neurons (Fig. 3 B and C).
Figure 2.
Ephrin-A5 inhibits the growth of neurites from medial thalamic neurons. Medial or lateral thalamic neurons growing on a confluent monolayer of ephrin-A5-expressing cells were detected after 48 hr in culture by immunostaining with anti-neuron-specific enolase. Neurons and their processes are stained darkly, and the underlying cells show light background staining. (A and B) Lateral thalamic neurons plated on control or ephrin-A5-expressing cells, respectively. Ephrin-A5 shows no significant effect on the neurite outgrowth of these neurons. (C and D) Medial thalamic neurons plated on the control or ephrin-A5-expressing cells, respectively. Ephrin-A5 dramatically reduces the length of neurites of these neurons. (Bar = 50 μm.)
Figure 3.
Quantitative analysis of ephrin-A5 effects on thalamic neurite outgrowth. (A) The length of neurites of medial thalamic neurons are significantly shorter when plated on the control cells than when plated on ephrin-A5-expressing cells. (B) Ephrin-A5 has little effect on the growth of neurites from the lateral thalamic neurons. (C) Average length (±SEM) of neurites on control or ephrin-A5-expressing cells. (D) The inhibitor EphA3-Fc abolishes the inhibitory effect of cell surface-anchored ephrin-A5 in dose-dependent manner. Soluble EphA3-Fc was used at 1 and 2 μg/ml. Control represents medial thalamic neurons grown on control-transfected NIH 3T3 cells. The absence of soluble ligand (0 μg/ml) on ephrin-transfected cells represents the condition in which the most potent inhibition of neurite outgrowth is obtained.
To confirm that the inhibition on the neurite outgrowth was specifically caused by ephrin-A5 expressed on the surface of the substrate cells, we investigated whether EphA3-Fc, the ligand-binding domain of EphA3 fused to the IgG Fc region, could reverse the inhibition by ephrin-A5. EphA3-Fc competes for binding of the ligand expressed on the 3T3 cell surface, thus preventing the interaction between the ligand and the receptors expressed on axons (27–29). As we predicted, the inhibitory effect of ephrin-A5 on neurite outgrowth was reduced significantly by the addition of EphA3-Fc to the coculture medium in a dose-dependent fashion (Fig. 3D).
Inhibition of Subicular Neurite Outgrowth by Ephrin-A5.
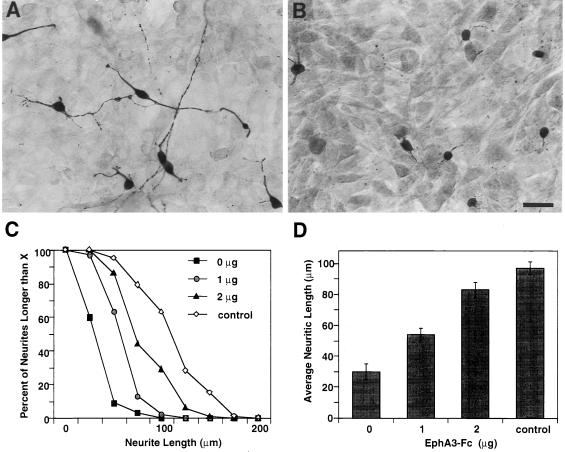
We next examined whether the inhibitory effect is specific for the medial thalamic neurons or is a more general effect for other developing neurons that have limbic projections. The effect of ephrin-A5 on neurite outgrowth was assayed by using limbic cortical neurons from the subiculum, which provides major output from the hippocampal formation to many limbic regions, including medial prefrontal and retrosplenial cortices (30, 31). The coculture assay showed that, similar to the medial thalamic neurons, subicular neurite outgrowth was reduced severely on NIH 3T3 cells expressing ephrin-A5 compared with control cells (Fig. 4 A and B). A 70% reduction of mean neurite outgrowth was observed (Fig. 4 C and D). Again, the inhibition was reduced by the addition of EphA3-Fc in a dose-dependent manner, suggesting that the effect is specifically caused by the presence of ephrin-A5.
Figure 4.
Ephrin-A5 inhibits neurite outgrowth of subicular neurons. (A) Subicular neurons grow extensive neurites when plated on control cells. (B) Subicular neurons generally lack extensive neurites when plated on ephrin-A5-expressing cells. (C) EphA3-Fc abolishes the inhibitory effect of ephrin-A5 on subicular neurite outgrowth. (D) Average neuritic length (±SEM) of subicular neurons is increased in the presence of EphA3-Fc. (Bar = 55 μm.)
DISCUSSION
The current study has examined the role of signaling through specific elements of the Eph receptor family in regulating the growth of neurites from neurons that comprise specific thalamocortical circuits. Our results demonstrate that the EphA5 receptor and its ligand, ephrin-A5, are expressed in complementary patterns in the thalamus and their cortical targets. In addition, ephrin-A5 specifically inhibits neurite outgrowth of receptor-rich, medial thalamic neurons from limbic nuclei but sustains neurite outgrowth of the lateral thalamic neurons from primary sensory and motor nuclei. Further analysis using hippocampal neurons indicated that ephrin-A5 inhibits primarily the growth of axons detected with antibody against the axon-specific marker τ (60–70% reduction in axonal length), although a mild inhibition of dentritic growth also was observed (≈20% reduction) (P.-P.G. and R.Z., unpublished results). Thus, ephrin-A5 is likely to inhibit primarily the axons of the medial thalamic neurons. These observations suggest that the A5 Eph family receptor and ligand may serve to control the ingrowth of limbic thalamic axons to the lateral perirhinal and medial prefrontal cortices by restricting growth into the sensorimotor cortex through repulsive interactions. Our observations that ephrin-A5 also inhibits neurite outgrowth of subicular neurons indicate that this ligand may serve as a general inhibitor to prevent limbic innervation of sensorimotor cortex.
EphA5 Receptor and Ephrin-A5 Ligand Expression and Thalamocortical Organization.
The anatomical relationship between the cerebral cortex and afferents arising from neurons in the thalamus is well documented (5). Axons from primary sensory and motor nuclei located in the lateral and posterior thalamus project to the appropriate sensory or motor areas in the cortex, and the specificity is established prenatally in the rodent (6, 23–25, 32, 33). In contrast, axons from more medial, limbic regions of the thalamus project to allocortical areas along the rhinal sulcus (4, 5, 34) and likely arise during early prenatal development as well (P.L., unpublished observations).
The present study, using in situ hybridization, documents a reciprocal relationship between the expression of EphA5 and ephrin-A5 in the thalamus and cerebral cortex, suggesting a role in generating specificity of thalamocortical projection during embryonic development. EphA5 expression is evident by embryonic day 18 in the medial thalamus and continues to be expressed in this pattern postnatally during the peak of thalamic ingrowth into the cortex and reciprocal projections from the cortex. Allocortical regions, which are the principal areas receiving input from the medial thalamic nuclei, express high levels of the EphA5 receptor (16) but very low levels of the complementary Eph ligand. Instead, cortical areas that are avoided by the medial thalamic axon groups, including primary motor and somatosensory areas, express the highest levels of ephrin-A5. The complementary expression of the Eph receptor and ligand in the limbic and nonlimbic regions of the cortex and the thalamus is analogous to that observed previously in the retinotectal and hippocamposeptal systems, consistent with their known roles as negative guidance cues in neuron–target interactions (10–15).
Ephrin-A5 Signaling Specifies Thalamic Neurite Outgrowth.
The prediction of growth-inhibiting effects of ephrin-A5 on medial thalamic neurons, which normally express high levels of EphA5, is supported by experiments in which the repulsive or growth-inhibiting activity of Eph-receptor activation has been demonstrated in the visual system or the hippocampal systems (10–13). In this study, neurite outgrowth of medial thalamic neurons was suppressed dramatically by ephrin-A5-expressing cells. Lateral thalamic neurons, which normally project into the sensorimotor cortex that expresses high levels of ephrin-A5, exhibit similar growth on the ligand-expressing cells and control-transfected cells. The reduction of subicular neurite outgrowth on ephrin-A5-expressing cells, predicted on the basis of high EphA5 expression in the subiculum and its normal projection to ephrin-A5-deficient prefrontal, prelimbic, and infralimbic cortices (30, 31), suggests that the Eph ligand establishes an inhibitory domain in the cerebral cortex to prevent limbic axons from innervating the sensorimotor cortices. The timing of expression of the Eph family receptor and ligand late in embryogenesis and early in the postnatal period is consistent with a role in maintaining restricted territories of ingrowth that parcelate basic limbic and nonlimbic organization.
We have examined the expression of all five known ligands of the EphA subfamily receptors; only ephrin-A5 has specific expression in the sensorimotor cortex. Therefore, ephrin-A5 may be the major contributor of the Eph signaling system in regulating thalamocortical projections. Ephrin-A5 interacts with multiple receptors of the EphA subfamily (35). Consequently, other receptors of the EphA subfamily, if expressed in the thalamus, also may participate in the regulation of thalamocortical projections.
Collaboration of Positive and Negative Guidance Cues Establishing Limbic and Nonlimbic Projections.
There is accumulating evidence that circuit formation may be mediated by interactions of distinct guidance cues that work coordinately to establish appropriate path-finding and target recognition. At least three families of growth-regulating molecules have been identified as candidates for the guidance signals in the developing cortex and thalamus. First, the Ig superfamily members such as the limbic-associated membrane protein (LAMP) and neurotrimmin are found in distinct limbic and primary sensory and motor regions, respectively (19, 36, 37), and have been shown to promote neurite outgrowth in a homophilic fashion in vitro (19, 38), indicating that positive guidance cues are essential for appropriate targeting of axon populations in the cortex. This notion is supported by in vivo transplant studies in which LAMP expression correlates precisely with the ability to form limbic thalamocortical (34) and cortico–cortical (39) connections of each graft with the host brain. Furthermore, LAMP has been shown to mediate targeting of septal and dentate gyrus neurons (19, 40).
Second, expression patterns of members of the cadherin family of adhesion molecules (Cad-6, -8, and -11) in the cerebral cortex were mapped to specific limbic and nonlimbic areas early postnatally (41). Although the function of cadherins in cortical development is not known, it is likely that areal organization and parcellation into general neo- and allocortical domains are influenced by the general promotion of cell–cell interactions by cadherins.
Third, the present study reveals that the Eph family members EphA5 and ephrin-A5 are expressed in a complementary fashion in thalamus and cortex. The inhibition reported here of neurite outgrowth by Eph receptor activation adds to the distinct families of guidance molecules that play critical roles in establishing topography in the central nervous system. The presence of both positive and negative guidance cues, distributed in an anatomically complementary fashion, fulfills a longstanding prediction of Sperry’s chemoaffinity theory (8, 9). The combinatorial strategy of establishing the specificity of axonal trajectories likely is to be a conserved mechanism. In Drosophila, for example, both Ig superfamily members (fasciclins) and semaphorins regulate pathway guidance and target selection during development (42). The relative roles of positive and negative cues in the establishment of thalamocortical projection patterns now can be addressed in vivo by manipulating expression patterns of different classes of molecules during defined periods of development when the pathways are established initially and proper target selection occurs de novo.
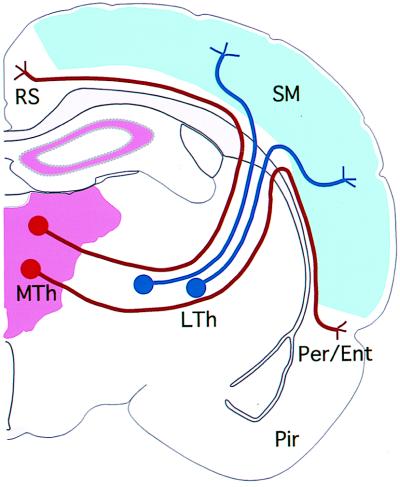
Figure 5.
Schematic diagram depicting the potential role of Eph ligand–receptor interaction in the generation of area specificity of thalamocortical projection. It is proposed that the medial thalamic neurons, which are EphA5-positive (shown in red), do not project to the sensorimotor cortex, which in part may be caused by the presence of ephrin-A5 in this region (shown in blue). Lateral thalamic neurons, in contrast, are not responsive to the presence of ephrin-A5 because they do not express the receptor. This is consistent with our observations that ephrin-A5 inhibits neurite outgrowth of the medial thalamic neurons but has no effect on neurite outgrowth from lateral thalamic neurons. It remains unclear what restricts the lateral neurons to the sensorimotor cortex and prevents them from innervating the limbic cortices.
Acknowledgments
This work was supported by a National Science Foundation grant (NSF IBN-9409930) and an Alzheimer’s Association grant to R.Z. (PRG-95–150) and by a National Institute of Mental Health grant (MH45507) to P.L.
References
- 1.Kass J H. Annu Rev Psychol. 1987;38:129–151. doi: 10.1146/annurev.ps.38.020187.001021. [DOI] [PubMed] [Google Scholar]
- 2.Levitt P, Barbe M F, Eagleson K L. Annu Rev Neurosci. 1997;20:1–24. doi: 10.1146/annurev.neuro.20.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Lopes de Silva F, Witter M P, Boeijinga P H, Lohman A H M. Physiol Rev. 1990;70:453–511. doi: 10.1152/physrev.1990.70.2.453. [DOI] [PubMed] [Google Scholar]
- 4.Price J L. In: The Rat Nervous System. Paxinos G, editor. San Diego: Academic; 1995. pp. 629–648. [Google Scholar]
- 5.Jones E G. The Thalamus. New York: Plenum; 1995. [Google Scholar]
- 6.DeCarlos J A, O’Leary D D M. J Neurosci. 1992;12:1194–1211. doi: 10.1523/JNEUROSCI.12-04-01194.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erzurumlu R S, Jhaveri S. Cereb Cortex. 1992;2:336–352. doi: 10.1093/cercor/2.4.336. [DOI] [PubMed] [Google Scholar]
- 8.Sperry R W. J Comp Neurol. 1943;79:33–55. [Google Scholar]
- 9.Sperry R W. Proc Natl Acad Sci USA. 1963;50:703–710. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drescher U, Kremoser C, Handwerker C, Loschinger J, Masaharu N, Bonhoeffer F. Cell. 1995;82:359–370. doi: 10.1016/0092-8674(95)90425-5. [DOI] [PubMed] [Google Scholar]
- 11.Gao P P, Zhang J H, Racey Y B, Dreyfus C F, Black I B, Zhou R. Proc Natl Acad Sci USA. 1996;93:11161–11166. doi: 10.1073/pnas.93.20.11161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamoto M, Cheng H-J, Friedman G C, McLaughlin T, Hansen M J, Yoon C H, O’Leary D D M, Flanagan J G. Cell. 1996;86:755–766. doi: 10.1016/s0092-8674(00)80150-6. [DOI] [PubMed] [Google Scholar]
- 13.Monschau B, Kremoser C, Ohta K, Tanaka H, Kaneko T, Yamada T, Handwerker C, Hornberger M R, Loschinger J, Pasquale E B, et al. EMBO J. 1997;16:1258–1267. doi: 10.1093/emboj/16.6.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng H-J, Nakamoto M, Bergemann A D, Flanagan J G. Cell. 1995;82:371–381. doi: 10.1016/0092-8674(95)90426-3. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J-H, Cerretti D P, Yu T, Flanagan J G, Zhou R. J Neurosci. 1996;16:7182–7192. doi: 10.1523/JNEUROSCI.16-22-07182.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J-H, Pimenta A F, Levitt P, Zhou R. Mol Brain Res. 1997;47:202–214. doi: 10.1016/s0169-328x(97)00051-x. [DOI] [PubMed] [Google Scholar]
- 17.Kozlosky C J, VandenBos T, Park L, Cerretti D P, Carpenter M K. Cytokine. 1997;9:540–549. doi: 10.1006/cyto.1997.0199. [DOI] [PubMed] [Google Scholar]
- 18.Lillian L. Nature (London) 1996;377:158–162. [Google Scholar]
- 19.Pimenta A F, Zhukareva V, Barbe M F, Reinoso B S, Grimley C, Henzel W, Fisher I, Levitt P. Neuron. 1995;15:287–297. doi: 10.1016/0896-6273(95)90034-9. [DOI] [PubMed] [Google Scholar]
- 20.Zhukareva V, Chernevskaya N, Pimenta A, Nowycky M. Mol Cell Neurosci. 1997;10:43–55. doi: 10.1006/mcne.1997.0639. [DOI] [PubMed] [Google Scholar]
- 21.Zhou R, Copeland T D, Kromer L F, Schulz N T. J Neurosci Res. 1994;37:129–143. doi: 10.1002/jnr.490370117. [DOI] [PubMed] [Google Scholar]
- 22.Lund R D, Mustari M J. J Comp Neurol. 1977;173:289–306. doi: 10.1002/cne.901730206. [DOI] [PubMed] [Google Scholar]
- 23.Wise S P, Jones E G. J Comp Neurol. 1978;178:187–208. doi: 10.1002/cne.901780202. [DOI] [PubMed] [Google Scholar]
- 24.Erzurumlu R S, Jhaveri S. Dev Brain Res. 1990;56:229–234. doi: 10.1016/0165-3806(90)90087-f. [DOI] [PubMed] [Google Scholar]
- 25.Catalano S M, Robertson R T, Killackey H P. Proc Natl Acad Sci USA. 1991;88:2999–3003. doi: 10.1073/pnas.88.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Leary D D M, Schlaggar B L, Tuttle R. Annu Rev Neurosci. 1994;17:419–439. doi: 10.1146/annurev.ne.17.030194.002223. [DOI] [PubMed] [Google Scholar]
- 27.Kozlosky C J, Maraskovsky E, McGrew J T, VandenBos T, Teepe M, Lyman S D, Srinivasan S, Fletcher F A, Gayle R B, III, Cerretti D P, et al. Oncogene. 1995;10:299–306. [PubMed] [Google Scholar]
- 28.Cerretti D P, VandenBos T, Nelson N, Kozlosky C J, Reddy P, Maraskovsky E, Park L S, Lyman SD, Copeland N G, Gilbert D J, et al. Mol Immunol. 1995;32:1197–1205. doi: 10.1016/0161-5890(95)00108-5. [DOI] [PubMed] [Google Scholar]
- 29.Lackmann M, Bucci T, Mann R J, Kravets L A, Viney E, Smith F, Moritz R L, Carter W, Simpson R J, Nicola N A, et al. Proc Natl Acad Sci USA. 1996;93:2523–2529. doi: 10.1073/pnas.93.6.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swanson L W, Kohler C, Bjorklund A. In: Handbook of Chemical Neuroanatomy. Bjorklund A, Hokfelt T, Swanson L W, editors. Vol. 5. Amsterdam: Elsevier; 1987. pp. 124–278. [Google Scholar]
- 31.Amaral D G, Witter M P. In: The Rat Nervous System. Paxino G, editor. San Diego: Academic; 1995. pp. 443–493. [Google Scholar]
- 32.Agmon A, Yang L T, Jones E G, O’Dowd D K. J Neurosci. 1995;15:549–561. doi: 10.1523/JNEUROSCI.15-01-00549.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghosh A, Shatz C J. Development (Cambridge, UK) 1993;117:1031–1047. doi: 10.1242/dev.117.3.1031. [DOI] [PubMed] [Google Scholar]
- 34.Barbe M F, Levitt P. Proc Natl Acad Sci USA. 1992;89:3706–3710. doi: 10.1073/pnas.89.9.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Pharmacol Ther. 1998;77:151–181. doi: 10.1016/s0163-7258(97)00112-5. [DOI] [PubMed] [Google Scholar]
- 36.Reinoso B S, Pimenta A F, Levitt P. J Comp Neurol. 1996;375:274–288. doi: 10.1002/(SICI)1096-9861(19961111)375:2<274::AID-CNE7>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 37.Pimenta A F, Reinoso E S, Levitt P. J Comp Neurol. 1996;375:289–302. doi: 10.1002/(SICI)1096-9861(19961111)375:2<289::AID-CNE8>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 38.Zhukareva V, Levitt P. Development (Cambridge, UK) 1995;121:1161–1172. doi: 10.1242/dev.121.4.1161. [DOI] [PubMed] [Google Scholar]
- 39.Barbe M F, Levitt P. J Neurosci. 1995;15:1819–1834. doi: 10.1523/JNEUROSCI.15-03-01819.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keller F, Rimvall K, Barbe M F, Levitt P. Neuron. 1989;3:551–561. doi: 10.1016/0896-6273(89)90265-1. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki S C, Inoue T, Kimura Y, Tanaka T, Takeichi M. Mol Cell Neurosci. 1997;9:433–447. doi: 10.1006/mcne.1997.0626. [DOI] [PubMed] [Google Scholar]
- 42.Goodman C. Annu Rev Neurosci. 1996;19:341–377. doi: 10.1146/annurev.ne.19.030196.002013. [DOI] [PubMed] [Google Scholar]