Abstract
The mothers of infant rats show individual differences in the frequency of licking/grooming and arched-back nursing (LG-ABN) of pups that contribute to the development of individual differences in behavioral responses to stress. As adults, the offspring of mothers that exhibited high levels of LG-ABN showed substantially reduced behavioral fearfulness in response to novelty compared with the offspring of low LG-ABN mothers. In addition, the adult offspring of the high LG-ABN mothers showed significantly (i) increased central benzodiazepine receptor density in the central, lateral, and basolateral nuclei of the amygdala as well as in the locus ceruleus, (ii) increased α2 adrenoreceptor density in the locus ceruleus, and (iii) decreased corticotropin-releasing hormone (CRH) receptor density in the locus ceruleus. The expression of fear and anxiety is regulated by a neural circuitry that includes the activation of ascending noradrenergic projections from the locus ceruleus to the forebrain structures. Considering the importance of the amygdala, notably the anxiogenic influence of CRH projections from the amygdala to the locus ceruleus, as well as the anxiolytic actions of benzodiazepines, for the expression of behavioral responses to stress, these findings suggest that maternal care during infancy serves to “program” behavioral responses to stress in the offspring by altering the development of the neural systems that mediate fearfulness.
The development of responses to stress in the rat is influenced by the early postnatal environment (for reviews see refs. 1–3). Thus, postnatal handling during the first week of life decreases behavioral fearfulness and hypothalamic–pituitary–adrenal (HPA) responses under conditions of stress. These effects persist throughout the life of the animal (4, 5) and form a basis for vulnerability to stress-related disease (6).
The handling paradigm reflects the remarkable plasticity that exists within neural systems that mediate responses to stress. The manipulation is actually rather subtle. Handled pups are simply removed from the nest for 3–15 min and then reunited with the mother. Handling does not represent a period of maternal deprivation, because over the course of the day mothers are routinely off their nests for periods of 20–25 min (see refs. 7–9). At the same time the artificial and nonspecific nature of the handling paradigm is unsettling (see refs. 10 and 11). Under natural conditions, development in the rat typically occurs in the rather dark, tranquil confines of a burrow where the major source of stimulation is that of the mother and littermates: there is little here that resembles the disruption associated with human handling. However, several studies have shown that postnatal handling actually alters the behavior of the mother toward her pups, leading to the idea that the effects of postnatal handling may be mediated by changes in mother–pup interactions (1, 12, 13).
In the Norway rat, mother–pup contact occurs primarily within the context of a nest-bout, which begins when the mother approaches the litter, gathers the pups under her, and licks/grooms the pups. The mother then nurses the suckling pups while continuing to occasionally lick/groom (see refs. 7–9, 14, 15). Mothers of handled (H) pups spend the same amount of time with their litters as do mothers of nonhandled (NH) pups; however, they have shorter, but more frequent, nest-bouts (B. C. Woodside, M.J.M., and J. Jans, unpublished observations) and spend significantly more time licking/grooming pups (see refs. 16 and 17) than do mothers of NH pups. This latter effect is likely because of the fact that mothers of H litters are getting on and off the pups more frequently, and licking/grooming is closely associated with the early phase of each bout. Mothers of H pups also showed substantially more arched-back nursing: mothers of NH pups more frequently adopt a “blanket” posture lying over pups (see ref. 18). These findings are consistent with the “maternal mediation” hypothesis (see ref. 13).
The critical question is: Are the effects of handling on maternal behavior related to the development of behavioral and endocrine responses to stress? We reasoned that if these differences in maternal behaviors are indeed critical, then the offspring of mothers that naturally show higher levels of licking/grooming and/or arched-back nursing should exhibit behavioral and endocrine responses to stress that resemble those of H animals. To test this idea, we examined maternal behavior from postnatal days 1–10 in a cohort of dams whose pups were unmanipulated, looking for individual differences in licking/grooming and arched-back nursing, because these behaviors serve to distinguish the behavior of mothers of H from those of NH pups. The offspring of mothers that showed high or low levels of licking/grooming and arched-back nursing (high vs. low LG-ABN) were then allowed to mature to adulthood and examined by using a number of tests of fear in the presence of novelty. We also examined levels of central benzodiazepine (BZ) (CBZ) receptor, α2 adrenoreceptors, and corticotropin-releasing hormone (CRH) receptor binding in the amygdala and the locus ceruleus, systems that are implicated in the expression of fearfulness (see refs. 19 and 20).
MATERIALS AND METHODS
Animals.
The animals were Long–Evans hooded rats obtained from Charles River Canada (St. Constant, Québec) and housed in 46 cm × 18 cm × 30 cm Plexiglas cages. Food and water were provided ad libitum. The colony was maintained on a 12:12 light:dark schedule with lights on at 0800. The animals underwent routine cage maintenance beginning on day 12 but were otherwise unmanipulated. All procedures were performed according to guidelines developed by the Canadian Council on Animal Care.
The behavior of each dam was observed (see ref. 21) for eight 60-min observation periods daily for the first 10 days postpartum. Observers were trained by using video tapes and still photography to a high level of inter-rater reliability (i.e., > 0.90). Observations were performed at six periods during the light phase (0800, 1000, 1100, 1430, 1600, and 1800) and two periods during the dark phase of the light:dark cycle (2000 and 0600). Within each observation period the behavior of each mother was scored every 4 min (15 observations per period × 8 periods per day = 120 observations per mother per day) for the following behaviors: mother off pups, mother carrying pup, mother licking and grooming any pup, mother nursing pups in an arched-back posture, a “blanket” posture in which the mother lays over the pups, or a passive posture in which the mother is lying on either her back or her side while the pups nurse (see ref. 19 for a description of behaviors).
At the time of weaning on day 22 of life the male offspring were housed in same-sex, same-litter groups of three or four animals per cage until day 45 of life, and two animals per cage from this point until the time of testing, which occurred no earlier than 100 days of age. All experiments were performed by individuals who were blind to the developmental history of the animals.
Behavioral Testing.
For the measures of novelty-induced suppression of appetitive behavior (see refs. 18 and 22), animals were food deprived for 24 hr prior to testing and then provided with food either in a novel environment or in the home cage. The novel environment was a 180 cm × 180 cm × 30 cm arena in a well-lit room with food provided in a cylindrical wire-mesh hopper located in the center of the novel arena. The test session lasted for 10 min, and during this period the experimenter scored the latency (sec) to begin feeding and the total amount of time spent feeding. If an animal did not eat within the test period, a score of 600 seconds was assigned for latency measures and 0 for the amount of time feeding. A separate group of animals was tested in the same manner with food provided in the home cage rather than in the novel environment.
Another set of animals were examined in an open-field test of exploration. Animals were placed, one at a time, in a novel, circular open field, 1.6 m in diameter. The critical measure was the time (sec) the animal spent exploring the inner area of the novel arena. Exploration was defined as the entire body of the animal being away from the immediate vicinity of the wall (>10 cm) enclosing the open field. Each rat was tested for 5 min in the novel environment. The open field was cleaned between each subject to prevent olfactory cues from affecting the behavior of subsequently tested rats.
In Vitro Receptor Autoradiography.
For all experiments, brains were obtained from adult (approximately 100 days of age) offspring of high or low LG-ABN mothers (n = 5 or 6 per group) by rapid decapitation less than 1 min after removal from the home cage. Brains were quickly removed, frozen in −70°C isopentane, and stored at −80°C. Brains were sectioned in the coronal plane at 15 μm, and sections were thaw-mounted onto gel-coated slides, which were then stored at −80°C until the time of assay.
For CBZ receptor assays (22) slides were thawed and preincubated in assay buffer (0.17 M Tris⋅HCl, pH 7.4) for 30 min at 4°C. The slides were then incubated with a saturating, 0.5 nM, concentration of [3H]flunitrazepam (84.5 Ci/mmol, New England Nuclear; 1 Ci = 37 GBq) in assay buffer for 60 min at 4°C. Nonspecific background binding was determined in parallel sections by using 1 μM clonazepam. Post-assay washes (twice for 30 sec) were performed with assay buffer. The sections were left to dry overnight and then were apposed to tritium-sensitive Ultrafilm (Amersham Canada, Montreal) along with 3H microscales for 14 days.
CRH receptor binding was determined (24) with 125I-labeled (on Tyr residues) ovine CRH (oCRH) (DuPont/NEN, Boston, MA) in assay buffer containing 50 mM Tris⋅HCl, pH 7.4, with 5 mM MgCl2, 2 mM EGTA, 0.1% BSA, 100 kallikrein inhibitor units/ml aprotinin, and 1 mM DTT. Nonspecific binding was measured as 125I-oCRH binding in the presence of unlabeled 10 μM oCRH. Slides were incubated for 60 min at 22°C, and the binding reaction was stopped with the addition of ice-cold 7.5% polyethylene glycol (1 ml) in 50 mM Tris⋅HCl buffer (pH 7.4). The slides were then washed (three times, 15 min each) in assay buffer, air dried, and apposed to Ultrafilm along with 125I standards for 10 days.
For α2 adrenoreceptor receptor binding assays (19) sections were thawed and preincubated in 170 mM Tris⋅HCl buffer (pH 7.6, containing 5 mM EDTA, 1 mM MgCl2, and 0.1% ascorbic acid) for 30 min at room temperature. After the preincubation, the sections were incubated with a saturating, 3 nM, concentration of [3H]clonidine (61.9 Ci/mmol, New England Nuclear) in assay buffer for 60 min at 22°C. Nonspecific binding was determined with the addition of 100 μM norepinephrine (Aldrich). Sections were then washed (twice, 5 min each) in 0.17 M Tris⋅HCl buffer (pH 7.6) at 4°C and dried under a stream of cold air. Autoradiograms were generated by apposing slides to tritium-sensitive Ultrafilm with the appropriate [3H] microscales for 90 days.
Autoradiograms were analyzed by obtaining optical densities (expressed as mean ± SEM in fmol/mg of protein) determined by computer-assisted densitometry using an MCID image analysis system (Imaging Research, St. Catherine’s, Ontario, Canada), low activity tritium standards, and the rat brain atlas of Paxinos and Watson (20). There were three or four sections used in the analysis for each animal, and the mean from these values was then used in the statistical analysis.
Statistical Analysis.
For each experiment there were 1–3 pups per litter representing 3–5 litters per group. No animals were used in more than one experiment. Thus litter size limited the number of animals included in any particular experiment. To control for any potential “litter” effects we analyzed the data both by subject and by litter. There were no differences between these two methods of analysis in the statistical outcomes for any of the experiments. For the sake of brevity, we provide the results of the analysis by subject. The behavioral data were analyzed by using Student’s t test for between-group comparisons. Autoradiographic data were analyzed by using a two-way (Group × Region) analysis of variance with Tukey post-hoc tests where appropriate. Correlational analysis was performed by using simple regression analysis.
RESULTS
Maternal Behavior.
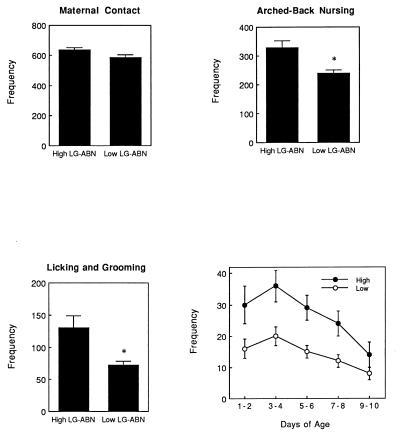
A portion of these data has been summarized in a previous report (16). There were stable naturally occurring individual differences in licking/grooming and in arched-back nursing posture. Because more than 90% of the instances of licking/grooming occurred while the mother was in the arched-back nursing posture, these two measures were highly correlated (r = +0.91). We then rank-ordered the dams on these measures and classified those mothers (n = 4) whose scores fell above the mean as high LG-ABN. The remaining dams (n = 5) were deemed low LG-ABN (see Fig. 1). The differences between high and low LG-ABN mothers were stable over the first 8 days of life for both licking/grooming (see Fig. 1) and arched-back nursing (data not shown). There were no differences between high vs. low LG-ABN mothers in the frequency of nursing or in the “mother on pups” category and likewise when the total of all categories involving mother–pup contact were combined to form a single measure of maternal contact, there were no differences between the groups (see Fig. 1). Thus, the variations in licking/grooming or arched-back nursing did not occur simply as a function of differences in the frequency of mother–pup contact. Moreover, the frequency of maternal LG-ABN was not significantly correlated with either litter size (range 9 to 15) or the gender composition (the ratio of males to females; range 0.67 to 1.6) of the litter (all r values < 0.15, not significant).
Figure 1.
Mean ± SEM frequency of maternal contact (the total of all behavioral categories involving contact between mother and pups), arched-back nursing, and licking/grooming in high versus low LG-ABN mothers over the first 10 days postnatally. The data represent the frequency of the behavior from a total of 1,200 observations per mother (120 observations per mother per day). ∗, P < 0.01. (Lower Right) Mean ± SEM frequency of licking/grooming over 2-day blocks for high versus low LG-ABN mothers. These data are derived from 240 observations per mother for each block. Differences between the groups were significant (P < 0.05) for days 1–8, but not on days 9–10.
Behavioral Studies.
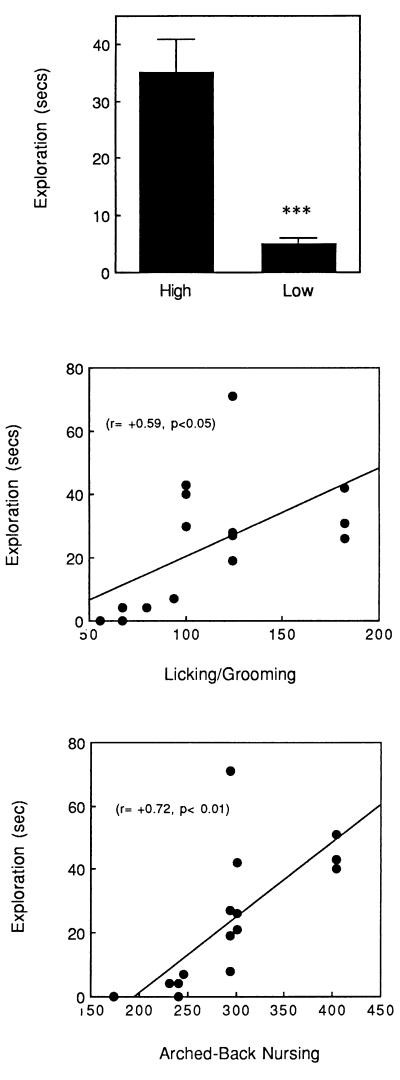
In the open-field test the adult offspring of high LG-ABN mothers showed significantly more exploration than did the offspring of low LG-ABN mothers (see Fig. 2). In addition, the time spent in exploration was significantly correlated (see Fig. 2) across animals with the frequency of both licking/grooming (r = +0.59, P < 0.05) and arched-back nursing (r = +0.72, P < 0.02).
Figure 2.
(Top) Mean ± SEM time spent in exploration of the inner area of an open field in the adult offspring of high versus low LG-ABN mothers. ∗∗∗, P < 0.001. The scattergrams depict the correlation between maternal licking/grooming (Middle) and arched-back nursing (Bottom) and exploration.
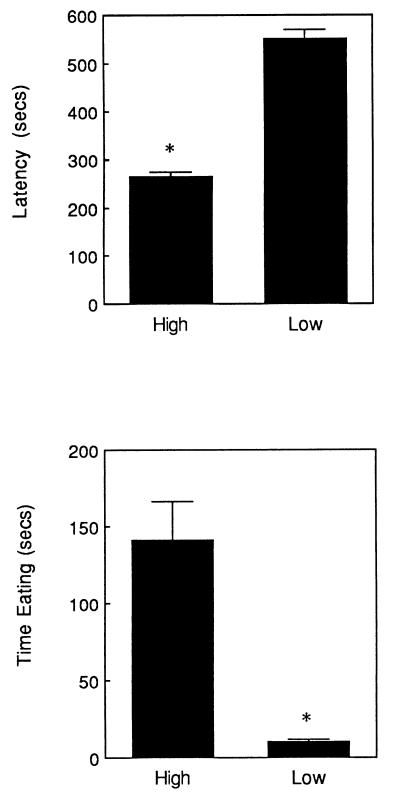
The offspring of high LG-ABN mothers showed a shorter latency to begin eating and spent more time eating in a novel environment than did the offspring of low LG-ABN mothers (see Fig. 3). In contrast to the behavioral differences in the novel testing situation, there were no differences between the groups on any of these measures when food was provided to the animals in the home cage. Under these conditions animals ate readily throughout the test period (the means for all groups were < 15 sec; data not shown). Thus, the differences in the latency to begin feeding were apparent only in the novel environment, suggesting that the relevant variable is the animals’ reaction to novelty, and not differences in appetite. As in the open-field test, there were significant correlations across animals between the various measures of fearfulness and maternal behavior. Thus, maternal licking/grooming was significantly correlated with both the latency to begin eating (r = +0.54, P < 0.05) and time spent eating (r = +0.70, P < 0.01). Likewise, maternal arched-back nursing was significantly correlated with the latency to begin eating (r = +0.59, P < 0.05) and time spent eating (r = +0.71, P < 0.01).
Figure 3.
Mean ± SEM latency to begin eating (Upper) and time eating (Lower) in a novel environment for the adult offspring of high (n = 10) versus low (n = 6) LG-ABN mothers. ∗, P < 0.01.
Receptor Autoradiography Studies.
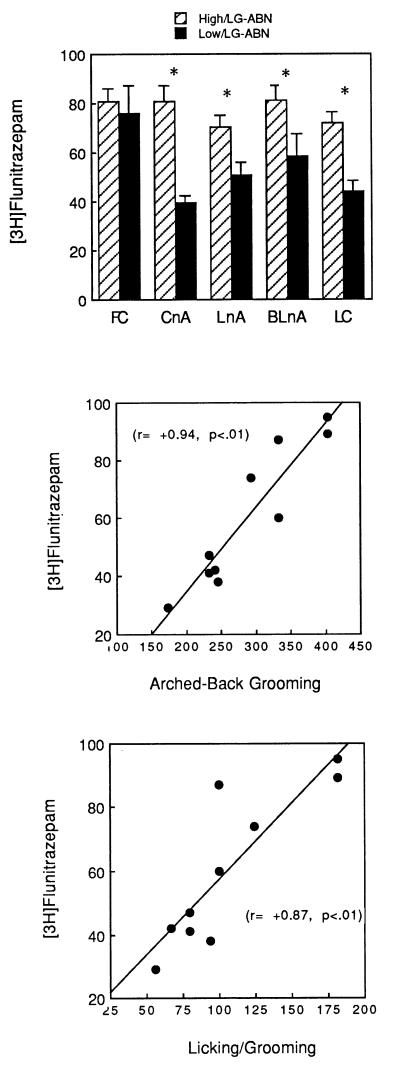
CBZ receptor density ([3H]flunitrazepam binding) was significantly higher in the offspring of high LG-ABN mothers in the central and lateral nuclei of the amygdala, as well as in the locus ceruleus (see Fig. 4). There were no differences in CBZ receptor levels in the frontal cortex or hippocampus (data not shown). We also correlated [3H]flunitrazepam binding levels in various brain regions to the frequency of maternal LG-ABN during the first 10 days of life. In the amygdaloid nuclei in particular, these correlations were striking. Thus, maternal licking/grooming was significantly correlated with [3H]flunitrazepam binding in both the central (r = +0.87, P < 0.01; see Fig. 4) and lateral (r = +0.81, P < 0.01) nuclei of the amygdala, as well as in the locus ceruleus (r = +0.63, P < 0.05). The same pattern was observed for the correlations between maternal arched-back nursing (central nucleus of the amygdala, r = +0.94, P < 0.01, see Fig. 3; lateral nucleus of the amygdala, r = +0.76, P < 0.01; locus ceruleus, r = +0.68, P < 0.05). The magnitude of these correlations in the amygdaloid nuclei was very high and the r2 value suggests that as much as three-quarters of the naturally occurring variation in CBZ receptor levels could be accounted for by variation in maternal care in early life.
Figure 4.
(Top) Mean ± SEM central benzodiazepine receptor ([3H]flunitrazepam) binding (in fmol/mg of protein) in the adult offspring of high versus low LG-ABN mothers. ∗, P < 0.01. The scattergrams depict the correlation between maternal licking/grooming (middle) and arched-back nursing (Bottom) and [3H]flunitrazepam binding in the central nucleus of the amygdala.
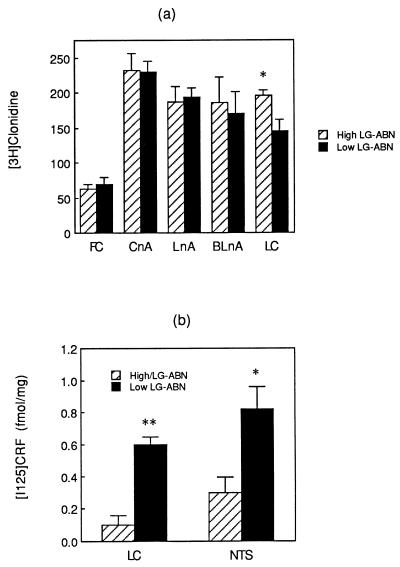
Levels of α2 adrenoreceptor binding were significantly higher in the locus ceruleus in the offspring of high compared with low LG-ABN mothers (see Fig. 5a). Moreover, α2 adrenoreceptor density in the locus ceruleus was significantly correlated with levels of both maternal licking/grooming (r = +0.73, P < 0.05) and arched-back nursing (r = +0.65, P < 0.05). There were no differences in any other brain region examined (see Fig. 5), reflecting a rather high degree of regional specificity.
Figure 5.
(a) Mean ± SEM α2 adrenoreceptor ([3H]clonidine) binding (in fmol/mg of protein) in the adult offspring of high versus low LG-ABN mothers. ∗, P < 0.05. (b) Mean ± SEM 125I-CRH binding (in fmol/mg of protein) in the adult offspring of high versus low LG-ABN mothers. ∗∗, P < 0.01.
CRH receptor binding was examined in the locus ceruleus and nucleus tractus solitarius, regions that are rich in noradrenergic cell bodies. CRH receptor binding was significantly higher in the offspring of the low LG-ABN mothers compared with those of high LG-ABN dams (see Fig. 5b). In both cases the levels of CRH receptor binding were significantly correlated with the frequency of maternal arched-back nursing (locus ceruleus, r = −0.82, P < 0.01; nucleus tractus solitarius, r = −0.66, P < 0.05). The correlations with maternal licking/grooming approached significance (locus ceruleus, r = −0.56, P < 0.10; nucleus tractus solitarius, r = −0.48, P < 0.10).
DISCUSSION
The critical issue for the maternal-mediation hypothesis is the functional equivalence between the effects of postnatal handling and those of increased maternal licking/grooming on behavioral and neuroendocrine responses to stress. The adult offspring of the high LG-ABN mothers showed significantly reduced behavioral fearfulness in comparison to the offspring of low LG-ABN mothers. Reduced fearfulness was the hallmark of the early postnatal handling studies (1–2). The offspring of the high vs. low LG-ABN mothers showed differences in CBZ, α2 adrenoreceptor, and CRH receptor binding in the amygdala–locus ceruleus/nucleus tractus solitarius system that mapped onto previously reported differences between H and NH rats (see Table 1). In addition, we (16) recently found that differences in HPA function between the offspring of high vs. low LG-ABN mothers are similar to those found in H vs. NH rats (also see Table 1). Taken together, these findings demonstrate a rather remarkable degree of similarity between the effects of increased maternal LG-ABN and those of postnatal handling. Moreover, in virtually each instance there was a significant correlation between the specific measure and the frequency of licking/grooming as well as arched-back nursing (see Results and ref. 16). These findings support the maternal-mediation hypothesis for the effects of postnatal handling. Thus, handling appears to alter the behavior of the mother toward the offspring, and these changes in mother–pup interactions appear to be the critical feature for the handling effect on the development of responses to stressors.
Table 1.
Summary of findings from handling (H vs. NH animals) and maternal observation studies (high vs. low LG-ABN mothers)
| Measure | Handling | Maternal LG-ABN |
|---|---|---|
| ACTH response to acute stress | H < NH | High < Low |
| CORT response to acute stress | H < NH | High < Low |
| Hippocampal GC receptor mRNA expression | H > NH | High > Low |
| PVNh CRF mRNA expression | H < NH | High < Low |
| GC negative-feedback sensitivity | H > NH | High > Low |
| Open-field exploration | H > NH | High > Low |
| Novelty-suppression of feeding | H > NH | High > Low |
| CBZ receptor | ||
| Central nucleus of Amygdala | H > NH | High > Low |
| Lateral nucleus of Amygdala | H > NH | High > Low |
| Locus ceruleus | H > NH | High > Low |
| Nucleus tractus solitarius | H > NH | High > Low |
| Hippocampus | H = NH | High = Low |
| Frontal cortex | H = NH | High = Low |
| Medial prefrontal cortex | H = NH | High = Low |
| CRF receptor | ||
| Locus ceruleus | H < NH | High < Low |
| α2 adrenoreceptor | ||
| Locus ceruleus | H > NH | High > Low |
| Nucleus tractus solitarius | H > NH | High > Low |
| PVNh | H = NH | High = Low |
Maternal care influenced the development of neural systems that have been implicated in the expression of fear, notably CBZ, CRH and α2 adrenoreceptor systems. These effects were highly specific to the amygdala - locus ceruleus. Although the data are correlational, there is a wealth of evidence for the idea that these changes in receptor levels are functionally related to the differences in fearfulness between the offspring of high vs. low LG-ABN mothers.
The central nucleus of the amygdala (CnA) has long been implicated in the expression of autonomic and behavioral responses to stress (reviewed in refs. 25 and 26). Lesions of the CnA block the excitatory effects of both conditioned and unconditioned fear on startle responses and the development of conditioned emotional responses (see ref. 26). Lesions of the CnA also attenuate many of the pathological indices associated with conditioned fear, such as ulceration (27), while chronic CnA stimulation can mimic these effects (25–27).
The CnA projects directly to brainstem regions containing noradrenergic cell bodies, including the nucleus tractus solitarius and the locus ceruleus (28–30), and these projections contain CRH (31). Thus, lesions to the CnA result in an 80–85% decrease in CRHir levels in the locus ceruleus (32). A critical feature of this system involves amygdaloid regulation of ascending noradrenergic systems which contain CRH receptors (24) and are associated with the expression of fear and anxiety (e.g., see refs. 33 and 34). Stress increases CRH mRNA levels in the amygdala (35) as well CRH immunoreactivity levels in the locus ceruleus (36, 37). Both stress and CRH infusion have been shown to increase the firing rates of locus ceruleus neurons (see ref. 18) as well as tyrosine hydroxylase expression (38). These effects are blocked by the CRH receptor antagonist α-helical CRH (35, 37–39). Microinjections of α-helical CRH into the locus ceruleus also attenuate novelty-induced suppression of exploratory behavior in the defensive withdrawal paradigm (40), stress-induced behavioral freezing (41), and stress-induced release of norepinephrine (42). Taken together, these data suggest that the CRH input from the amygdala to the midbrain noradrenergic cell body regions and the subsequent activation of ascending noradrenergic projections provides a major neural circuit for the expression of fear-related behaviors. Thus, the increased CRH receptor binding in the locus ceruleus and nucleus tractus solitarius in the offspring of the low LG-ABN mothers is consistent with the finding of increased fearfulness in these animals.
Activity within the noradrenergic cell bodies is also subject to inhibitory feedback regulation via somatodendritic α2 adrenoreceptors (see ref. 43). Thus, α2 adrenoreceptor agonists decrease the firing rate of locus ceruleus neurons and norepinephrine release at terminal sites. The increased α2 adrenoreceptor density in the locus ceruleus of the offspring of the high LG-ABN mothers may therefore provide another mechanism for the decreased fearfulness in these animals.
Maternal LG-ABN was also associated with increased CBZ receptor binding in the amygdala as well as the locus ceruleus. Both the lateral and central nuclei of the amygdala have been proposed as critical sites for the anxiolytic effects of BZ agonists. Thus, the direct administration of CBZ receptor agonists into the amygdala yields an anxiolytic effect (44). Interestingly, the CRH neurons within the amygdala, which appear to mediate the expression of fearfulness, are a major target for benzodiazepine effects. The CBZ receptor agonist alprazolam reduces CRH content in the locus ceruleus (37). Moreover, de Boer et al. (45) have shown that BZ administration attenuates the anxiogenic effects of intracerbroventricular CRH treatment (also see ref. 46), which probably reflects a downstream effect on locus ceruleus neurons.
Considering the importance of this amygdaloid–locus ceruleus–CRH system in mediating behavioral responses to novelty (36, 39, 47), we propose that BZ–CRH interactions within the amygdala as well as BZ effects at the level of noradrenergic cell body regions are critical neural substrates for the differences in the response to novelty observed between the adult offspring of high vs. low LG-ABN mothers. Thus, increased maternal LG-ABN decrease fearfulness, in part at least, by decreasing target tissue sensitivity to CRH, while increasing sensitivity to BZs in the amygdala as well as to both BZs and α2 agonists in the noradrenergic cell body regions. The implicit assumption here for the relevance of the CBZ receptor difference is that there exists an endogenous BZ receptor agonist. This idea is consistent with the finding that RO15–1788, a CBZ receptor antagonist, reinstates fearfulness to novelty in habituated animals (18) and attenuates the behavioral differences to novelty in H and NH rats (48). The results of human clinical studies are also of relevance. Glue et al. (49) found that subjects that were high on neuroticism showed reduced sensitivity to the BZ midazolam. Roy-Byrne and colleagues (50, 51) found reduced sensitivity to diazepam in patients with panic disorders and proposed that the reduced CBZ sensitivity was related to the expression of anxiety. These findings are consistent with the idea that decreased CBZ receptor levels are associated with an increased expression of fear and anxiety, such as occurred in the offspring of the low LG-ABN mothers.
It is not clear how licking/grooming and/or arched-back nursing regulate these neural systems. However, maternal licking influences central nervous system development (14, 15, 52, 53), and regular tactile stimulation from the mother is an essential feature of pup development, serving to regulate physiology in a manner most conducive to somatic and neural growth. Our findings suggest that this stimulation in the neonate is also an important mediator of the effects of maternal care on long-term development in the offspring.
The origins of the individual differences in maternal care are not clear. It is possible that these differences in maternal behavior reflect individual differences in fearfulness in the dams themselves, in which case these findings might reflect an example of a nongenomic mode of inheritance. We cannot completely exclude a possible genetic mode of inheritance; however, two lines of evidence argue for the importance of maternal behavior as a critical variable. First, neonatal handling produces exactly the same profile of behavioral, pharmacological, and endocrine responses to stress as those observed in the offspring of high LG-ABN mothers. Handling also significantly increases maternal LG-ABN. Simply put, the mothers of H pups are high LG-ABN mothers regardless of their genetic background (see ref. 18), and the H pups then resemble the offspring of high LG-ABN mothers. Interestingly, adoption in early life increases maternal licking/grooming of pups and, as adults, the offspring resemble H animals (54). Maternal behavior is regulated by stimuli associated with the pups (see refs. 14 and 15). Handling increases pup vocalizations, which have, in turn, been associated with increased maternal licking/grooming (see ref. 55). This finding might well underlie the increased licking/grooming observed in the mothers of H pups (see refs. 16 and 17). Second, we (56, 57) have examined the potential effects of maternal behavior on the development of behavior and endocrine responses to stress in BALB/c mice, normally a strain that is very fearful and shows elevated HPA responses to stress. However, BALB/c mice cross-fostered to C57 mothers are significantly less fearful, with lower HPA responses to stress. Importantly, C57 mothers lick and groom about twice as frequently as BALB/c mothers. If the genetic influence was paramount, then we would expect no such relationship between maternal behavior and phenotype. We do not dispute the likelihood of a polygenetic influence on the development of fearfulness. However, these data suggest that the emergence of fearfulness occurs, in part at least, as a function of parental care, and thus a nongenomic mode of inheritance.
We believe that the effects of maternal care on the development of behavioral and endocrine responses to stress reflect a naturally occurring plasticity whereby the behavior of the mother results in the programming of rudimentary responses to threatening stimuli. Like humans, the Norway rat inhabits a tremendous variety of ecological niches, each with varied sets of environmental demands. This plasticity could allow animals to adapt defensive systems to the unique demands of the environment. Because most mammals usually spend their adult life in an environment that is either the same or quite similar to that in which they were born, developmental “programming” of central nervous system responses to stress in early life is likely to be of adaptive value to the adult (see ref. 22). Such programming affords the animal an appropriate behavioral response, minimizing the need for a long and perhaps unaffordable period of adaptation in adult life. The mother’s behavior serves as the major link between the pup and its habitat (14, 15, 52), and thus serves as a very reasonable source of “environmental information” for the programming of neural systems that mediate behavioral and endocrine responses to stress.
Acknowledgments
This research was supported by grants from the Medical Research Council of Canada (M.J.M.) and from the National Institute of Mental Health (P.M.P., M.J.M.).
ABBREVIATIONS
- HPA
hypothalamic–pituitary–adrenal
- CRH
corticotropin-releasing hormone
- oCRH
ovine CRH
- BZ
benzodiazepine
- CBZ
central BZ
- H
handled
- NH
nonhandled
- LG-ABN
licking/grooming–arched-back nursing
- CnA
central nucleus of the amygdala
References
- 1.Levine S. In: Society, Stress and Disease. Levi L, editor. London: Oxford Univ. Press; 1975. pp. 43–50. [Google Scholar]
- 2.Denenberg V H. Psychol Rev. 1964;71:335–351. doi: 10.1037/h0042567. [DOI] [PubMed] [Google Scholar]
- 3.Meaney M J, Diorio J, Widdowson J, LaPlante P, Caldji C, Seckl J R, Plotsky P M. Dev Neurosci. 1996;18:49–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- 4.Meaney M J, Aitken D H, Bhatnagar S, Van Berkel C, Sapolsky R M. Science. 1988;238:766–770. doi: 10.1126/science.3340858. [DOI] [PubMed] [Google Scholar]
- 5.Vallée M, Mayo W, Dellu F, Le Moal M, Simon H, Maccari S. J Neurosci. 1996;17:2626–2636. doi: 10.1523/JNEUROSCI.17-07-02626.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seckl J R, Meaney M J. Lancet. 1993;342:1236. doi: 10.1016/0140-6736(93)92215-f. [DOI] [PubMed] [Google Scholar]
- 7.Leon M, Croskerry P G, Smith G K. Physiol Behav. 1978;21:793–811. doi: 10.1016/0031-9384(78)90021-5. [DOI] [PubMed] [Google Scholar]
- 8.Jans J, Woodside B C. Dev Psychobiol. 1990;23:519–532. doi: 10.1002/dev.420230607. [DOI] [PubMed] [Google Scholar]
- 9.Fleming A, Rosenblatt J S. Behav Neurosci. 1974;86:221–232. [Google Scholar]
- 10.Daly M. Br J Psychol. 1972;64:435–460. doi: 10.1111/j.2044-8295.1973.tb01370.x. [DOI] [PubMed] [Google Scholar]
- 11.Alberts J R. In: Developmental Neuropsychobiology. Greenough W T, editor. Orlando: Academic; 1986. pp. 117–137. [Google Scholar]
- 12.Barnett S A, Burn J. Nature (London) 1967;213:150–152. doi: 10.1038/213150a0. [DOI] [PubMed] [Google Scholar]
- 13.Smotherman W P, Bell R W. In: Maternal Influences and Early Behavior. Bell R W, Smotherman W P, editors. New York: Spectrum; 1980. pp. 201–210. [Google Scholar]
- 14.Alberts J R, Cramer C P. In: Handbook of Behavioral Neurobiology. Blass E M, editor. Vol. 9. New York: Plenum; 1988. pp. 1–39. [Google Scholar]
- 15.Hofer M A. In: Symbiosis in Parent-Offspring Interactions. Rosenblum L A, Moltz H, editors. New York: Plenum; 1983. pp. 61–75. [Google Scholar]
- 16.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky P M, Meaney M J. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 17.Lee M H S, Williams D I. Anim Behav. 1974;22:679–681. [Google Scholar]
- 18.Bodnoff S R, Suranyi-Cadotte B E, Quirion R, Meaney M J. Behav Neurosci. 1989;103:209–212. doi: 10.1037//0735-7044.103.1.209. [DOI] [PubMed] [Google Scholar]
- 19.Young W S, III, Kuhar M J. Proc Natl Acad Sci. 1980;77:1696–1700. doi: 10.1073/pnas.77.3.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paxinos G, Watson D. The Rat Brain in Stereotaxic Coordinates. New York: Academic; 1982. [Google Scholar]
- 21.Myers M M, Brunelli S A, Shair H N, Squire J M, Hofer M A. Dev Psychobiol. 1989;22:55–67. doi: 10.1002/dev.420220105. [DOI] [PubMed] [Google Scholar]
- 22.Britton D R, Thatcher-Britton R. Pharmacol Biochem Behav. 1981;15:577–582. doi: 10.1016/0091-3057(81)90212-4. [DOI] [PubMed] [Google Scholar]
- 23.Bureau M H, Olsen R W. J Neurochem. 1993;61:1479–1491. doi: 10.1111/j.1471-4159.1993.tb13643.x. [DOI] [PubMed] [Google Scholar]
- 24.DeSouza E B, Insel T R, Perrin M H, Rivier J, Vale W W, Kuhar M J. J Neurosci. 1985;5:3189–3202. doi: 10.1523/JNEUROSCI.05-12-03189.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LeDoux J E. In: The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. Aggleton J P, editor. New York: Wiley-Liss; 1992. pp. 339–351. [Google Scholar]
- 26.Davis M. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 27.Henke P G. Brain Res Bull. 1983;10:833–840. doi: 10.1016/0361-9230(83)90216-2. [DOI] [PubMed] [Google Scholar]
- 28.Van der Kooy D, Koda L Y, McGinty J F, Gerfen C R, Bloom F E. J Comp Neurol. 1984;224:1–24. doi: 10.1002/cne.902240102. [DOI] [PubMed] [Google Scholar]
- 29.Gray T S, Carney M E, Magnuson D J. Neuroendocrinology. 1989;50:433–446. doi: 10.1159/000125260. [DOI] [PubMed] [Google Scholar]
- 30.Moga M M, Gray T S. J Comp Neurol. 1985;241:275–284. doi: 10.1002/cne.902410304. [DOI] [PubMed] [Google Scholar]
- 31.van Bockstaele E J, Colago E E O, Valentino R J. J Comp Neurol. 1996;364:523–534. doi: 10.1002/(SICI)1096-9861(19960115)364:3<523::AID-CNE10>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 32.Koegler-Muly S M, Owens M J, Kilts G N E D, Nemeroff C B. J Neuroendocrinology. 1993;5:95–98. doi: 10.1111/j.1365-2826.1993.tb00367.x. [DOI] [PubMed] [Google Scholar]
- 33.Valentino R J, Aston-Jones G S. In: Psychopharmacology: The Fourth generation of Progress. Bloom F E, Kupfer D J, editors. New York: Raven; 1993. pp. 373–385. [Google Scholar]
- 34.Redmond D E, Huang Y H, Baulu J, Gold M S. In: Catecholamines: Basic and Clinical Frontiers. Usdiin E, Kopin I, Barchas J, editors. New York: Pergamon; 1979. pp. 1693–1695. [Google Scholar]
- 35.Kalin N H, Takahashi L K, Chen F-L. Brain Res. 1994;656:182–186. doi: 10.1016/0006-8993(94)91382-x. [DOI] [PubMed] [Google Scholar]
- 36.Owens M J, Nemeroff C B. Pharmacol Rev. 1991;43:425–473. [PubMed] [Google Scholar]
- 37.Owens M J, Vargas M A, Knight D L, Nemeroff C B. J Pharmacol Exp Ther. 1990;258:349–356. [PubMed] [Google Scholar]
- 38.Melia K R, Duman R S. Proc Natl Acad Sci USA. 1991;88:8382–8386. doi: 10.1073/pnas.88.19.8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dunn A J, Berridge C W. Brain Res Rev. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- 40.Butler P D, Weiss J M, Stout J C, Nemeroff C B. J Neurosci. 1990;10:176–183. doi: 10.1523/JNEUROSCI.10-01-00176.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swiergiel A H, Takahashi L K, Kalin N H. Brain Res. 1993;623:229–234. doi: 10.1016/0006-8993(93)91432-r. [DOI] [PubMed] [Google Scholar]
- 42.Lavicky J, Dunn A J. J Neurochem. 1993;60:602–612. doi: 10.1111/j.1471-4159.1993.tb03191.x. [DOI] [PubMed] [Google Scholar]
- 43.Simson P E, Weiss J M. J Neurosci. 1987;7:1732–1740. doi: 10.1523/JNEUROSCI.07-06-01732.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hodges H, Green S, Glenn B. Psychopharmacology. 1987;92:491–504. doi: 10.1007/BF00176484. [DOI] [PubMed] [Google Scholar]
- 45.de Boer S F, Katz J L, Valentino R J. J Pharmacol Exp Ther. 1992;262:335–342. [PubMed] [Google Scholar]
- 46.Swerdlow N R, Geyer M A, Vale W W, Koob G F. Psychopharmacology (Berlin) 1986;88:147–152. doi: 10.1007/BF00652231. [DOI] [PubMed] [Google Scholar]
- 47.Liang K C, Melia K R, Campeau S, Falls W A, Miserendino M J D, Davis M. J Neurosci. 1992;12:2313–2320. doi: 10.1523/JNEUROSCI.12-06-02313.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Escorihuela R M, Fernandez-Teruel A, Núñez F J, Zapata A, Tobena A. Psychopharmacology (Berlin) 1992;106:282–284. doi: 10.1007/BF02801985. [DOI] [PubMed] [Google Scholar]
- 49.Glue P, Wilson S, Coupland N, Ball D, Nutt D. J Anxiety Disorders. 1995;9:33–45. [Google Scholar]
- 50.Roy-Byrne P, Cowley D S, Greenblatt D J, Shader R I, Hommer D. Arch Gen Psychiatry. 1990;47:534–538. doi: 10.1001/archpsyc.1990.01810180034006. [DOI] [PubMed] [Google Scholar]
- 51.Roy-Byrne P, Wingerson D K, Radant A, Greenblatt D J, Cowley D S. Am J Psychiatry. 1996;153:1444–1449. doi: 10.1176/ajp.153.11.1444. [DOI] [PubMed] [Google Scholar]
- 52.Levine S. Ann N Y Acad Sci. 1994;746:260–275. doi: 10.1111/j.1749-6632.1994.tb39245.x. [DOI] [PubMed] [Google Scholar]
- 53.Schanberg S M, Field T M. Child Dev. 1987;58:1431–1447. [PubMed] [Google Scholar]
- 54.Barbazanges A, Vallée M, Mayo W, Day J, Simon H, Le Moal M, Maccari S. J Neurosci. 1996;16:7783–7790. doi: 10.1523/JNEUROSCI.16-23-07783.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bell R W, Nitschke W, Gorry T H, Zachma T. Dev Psychobiol. 1971;4:181–197. doi: 10.1002/dev.420040209. [DOI] [PubMed] [Google Scholar]
- 56.Zaharia M D, Shanks N, Meaney M J, Anisman H. Psychopharmacology (Berlin) 1996;128:227–239. doi: 10.1007/s002130050130. [DOI] [PubMed] [Google Scholar]
- 57.Anisman, H., Zaharia, M. D., Meaney, M. J. & Merali, Z. (1998) Int. J. Dev. Neurosci., in press. [DOI] [PubMed]