Abstract
In the primate cerebral cortex, morphologically and functionally diverse classes of local circuit neurons containing the inhibitory neurotransmitter γ-aminobutyric acid (GABA) differentially regulate the activity of pyramidal cells, the principal type of excitatory output neurons. In schizophrenia, GABA neurotransmission in the prefrontal cortex (PFC) appears to be disturbed but whether specific populations of GABA neurons are affected is not known. The chandelier class of GABA neurons are of particular interest because their axon terminals, which form distinctive arrays termed “cartridges,” provide inhibitory input exclusively to the axon initial segment of pyramidal cells. Thus, chandelier cells are positioned to powerfully regulate the excitatory output of pyramidal neurons and, consequently, to substantially affect the patterns of neuronal activity within the PFC. In this study, an antibody directed against the GABA membrane transporter GAT-1 was used to label GABA axon terminals in postmortem human brains. The relative density of GAT-1-immunoreactive axon cartridges furnished by chandelier neurons was decreased by 40% in the PFC of schizophrenic subjects compared with matched groups of normal control and nonschizophrenic psychiatric subjects. In contrast, markers of the axon terminals of other populations of GABA neurons were not altered in the schizophrenic subjects. Furthermore, the density of GAT-1-immunoreactive axon cartridges was not altered in psychiatric subjects who had been treated with antipsychotic medications. The changes in GAT-1-immunoreactive axon cartridges of chandelier neurons in schizophrenia are likely to reflect altered information processing within the PFC and in its output connections to other brain regions and could contribute to the cognitive impairments seen in this disorder.
Disturbances in certain cognitive processes are among the most disabling and persistent symptoms of schizophrenia (1), and these symptoms appear to be related to dysfunction of the dorsolateral portions of the prefrontal cortex (PFC) (2, 3). Although this dysfunction probably involves abnormalities in multiple components of PFC circuitry, a number of postmortem studies have reported alterations in cortical γ-aminobutyric acid (GABA) neural systems in subjects with schizophrenia. For example, both the uptake (4, 5) and release (6) of GABA have been reported to be reduced in cortical synaptosomes prepared from schizophrenic subjects. In the PFC, the activity of glutamic acid decarboxylase, the synthetic enzyme for GABA, is reduced in subjects with schizophrenia (6, 7), as is the expression of the mRNA for this enzyme (8). Finally, ligand binding studies have reported abnormalities in PFC GABAA receptors in schizophrenia (9). Although such changes in GABA neurotransmission are likely to be associated with substantial shifts in PFC function, determining the pathophysiological causes and consequences of altered GABA neurotransmission requires an understanding of which subpopulations of GABA neurons are affected.
In the primate PFC, GABA-containing local circuit neurons can be divided into at least a dozen morphologically and biochemically distinct subclasses (10), with each subclass relatively specialized in terms of its synaptic targets. The chandelier subpopulation of GABA neurons are of particular interest because their axon terminals, which form distinctive arrays termed “cartridges,” provide inhibitory input exclusively to the axon initial segment of pyramidal cells (11), the principal type of cortical excitatory projection neuron (12). Due to the location of the synapses formed by their axon terminals, chandelier cells are positioned to powerfully regulate the excitatory output of pyramidal neurons and, consequently, to substantially affect patterns of neuronal activity both within the PFC and in the numerous cortical and subcortical regions that receive projections from the PFC. Consequently, alterations in chandelier neurons in schizophrenia could account, at least in part, for the functional abnormalities observed in the PFC and interconnected brain regions in schizophrenia.
Antibodies directed against the GABA membrane transporter GAT-1 appear to label all GABAergic axon terminals in the cortex (13), including the distinctive axon cartridges of chandelier neurons. Thus, to test the hypothesis that alterations in chandelier neuron axon cartridges contribute to PFC dysfunction in schizophrenia, we examined the density, laminar distribution, and size of cartridge-like profiles with detectable levels of GAT-1 immunoreactivity in schizophrenic subjects and two matched comparison groups.
METHODS AND MATERIALS
Subjects.
Brain specimens from 55 subjects were obtained from the Allegheny County Coroner’s Office, with the consent of the next of kin and the approval of the Health Sciences Institutional Review Board of the University of Pittsburgh. Consensus DSM-III-R diagnoses were made by a group of four experienced clinicians with information obtained from medical records and structured interviews with family members (14). We studied 15 schizophrenic subjects, each of whom was matched to one normal control subject and one nonschizophrenic psychiatric subject on the basis of age, sex, and postmortem interval (Table 1). In addition, subject groups did not significantly differ in mean age of onset or duration of illness, tissue storage time, or incidence of out-of-hospital deaths, although the number of deaths by suicide was significantly higher in the psychiatric subjects than in the schizophrenic subjects (Table 1). Three of the psychiatric subjects were receiving treatment with antipsychotic medications at the time of death. No neuropathological abnormalities were detected in the PFC with the following exceptions. Thioflavin S staining revealed a few neuritic plaques in one schizophrenic (triad 7; see Fig. 2), two normal control (triads 4 and 5), and two psychiatric (triads 7 and 9) subjects. In addition, a rare neurofibrillary tangle was observed in one psychiatric subject (triad 7), but none of these cases had a clinical history of dementia.
Table 1.
Demographic characteristics and densities of GAT-1-immunoreactive axon cartridges in the three subject groups
| Subject group
|
Analysis | |||
|---|---|---|---|---|
| Control | Schizophrenic | Psychiatric | ||
| Male/female ratio | 10:5 | 10:5 | 10:5 | P = NS |
| Age, years | 53.9 ± 13.8 | 53.6 ± 13.0 | 53.7 ± 12.4 | P = NS |
| Postmortem interval, h | 11.3 ± 5.4 | 11.7 ± 5.6 | 11.6 ± 5.2 | P = NS |
| Age at onset of illness, years* | NA | 26.5 ± 7.2 | 34.3 ± 15.4 | P = NS |
| Duration of illness, years* | NA | 26.7 ± 12.4 | 16.9 ± 12.5 | P = 0.097 |
| % suicide | 0.0 | 13.3 | 40.0 | P = 0.014 |
| % out-of-hospital deaths | 93.3 | 80.0 | 80.0 | P = NS |
| Tissue storage, months | 52.3 ± 18.3 | 54.9 ± 22.4 | 44.4 ± 25.7 | P = NS |
| Cartridge density, mm−2 | ||||
| Area 9 | 37.9 ± 17.0 | 20.9 ± 15.3 | 36.2 ± 9.3 | F2, 39 = 9.24, P = 0.0005 |
| Area 46 | 43.8 ± 16.0 | 26.0 ± 14.3 | 41.3 ± 12.2 | F2, 39 = 8.35, P = 0.001 |
All values are the mean ± SD. NS, not significant; NA, not applicable.
Because the available data were insufficient to determine the age of onset of three psychiatric subjects, the schizophrenic and psychiatric subjects from these triads (patients 2, 5, and 6) were not included in these analyses.
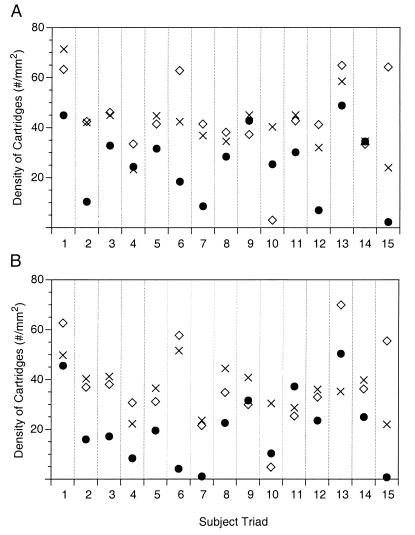
Figure 2.
Mean cartridge density in individual triads of subjects in areas 9 (A) and 46 (B). ◊, Normal control subjects; ×, nonschizophrenic psychiatric subjects; •, schizophrenic subjects. The psychiatric subjects in triads 3, 10, and 15 had been treated with antipsychotic medications, and the schizophrenic subjects in triads 5 and 7 were not on these medications at the time of death.
To assess the possible influence of antipsychotic medications on the measures of interest, we also studied five additional nonschizophrenic psychiatric subjects, who had been treated with these medications, and their matched normal controls. The demographic characteristics and diagnoses for all eight pairs of antipsychotic-treated psychiatric and normal control subjects are shown in Table 2.
Table 2.
Demographic characteristics and densities of GAT-1-immunoreactive axon cartridges in psychiatric subjects treated with antipsychotic medications and matched controls
| Subject group
|
||
|---|---|---|
| Control | Psychiatric* | |
| Male/female ratio | 5:3 | 5:3 |
| Age, years | 58.4 ± 17.4 | 58.0 ± 14.7 |
| Postmortem interval, h | 11.8 ± 6.2 | 11.3 ± 5.2 |
| Tissue storage, months | 61.8 ± 18.7 | 55.1 ± 33.6 |
| Cartridge density, mm−2 | ||
| Area 9 | 32.5 ± 19.4 | 28.8 ± 16.6 |
| Area 46 | 38.0 ± 21.1 | 37.4 ± 16.7 |
Groups were not significantly different on any measure; all values are the mean ± SD.
DSM-IIIR diagnoses: Major depression with psychotic features (n = 4), bipolar disorder with psychotic features (n = 1), organic affective disorder with psychotic features (n = 2), organic delusional disorder (n = 1).
Tissue Preparation.
For each subject, coronal blocks (1.0 cm thick) from the left PFC were immersed in ice-cold 4% paraformaldehyde in phosphate buffer for 48 h, washed in a graded series of sucrose solutions, and then stored in an antifreeze solution at −30°C. Previous studies have demonstrated that storage under these conditions does not alter immunoreactivity (14, 15).
GAT-1 Immunocytochemistry.
Tissue blocks were cut (40 μm) on a cryostat and every tenth section was stained for Nissl substance. These sections were used to identify the location of PFC areas 9 and 46 by cytoarchitectonic criteria (16, 17). Free-floating sections containing areas 9 and 46 were treated with 1% hydrogen peroxide for 15 min to eliminate endogenous peroxidase activity, rinsed in PBS, and then incubated in PBS containing 0.3% Triton X-100 and 5% normal goat serum for 30 min at room temperature. Sections were then incubated in PBS containing 0.3% Triton X-100, 3.5% normal goat serum, 0.05% BSA, and either a rabbit anti-GAT-1 antibody (1:1,000 dilution, Chemicon, Temecula, CA) or a rabbit anti-calretinin antibody (1:3,000 dilution, SWant, Bellinzona, Switzerland) for 2 days at 4°C, and processing was completed by using the Vectastain ABC kit (Vector Laboratories) and diaminobenzidine. Sections from each triad or pair of subjects were processed together through all steps and were then mounted on coded slides.
The specificity of the anti-GAT-1 antibody has been described (13). In addition, replacement of the primary antibody with normal goat serum produced an absence of immunoreactivity, and incubation of sections with antibodies directed against GAT-2 or GAT-3 (Chemicon) produced different patterns of immunoreactivity without labeled axon cartridges. The specificity of the anti-calretinin antibody was confirmed by preadsorption experiments with cognate and noncognate proteins as described (16, 18).
Quantification of GAT-1-Immunoreactive Axon Cartridges.
Quantification was performed without knowledge of subject number or diagnosis in both areas 9 and 46. On each tissue section, all GAT-1-immunoreactive axon cartridges within 500-μm-wide traverses extending from the pial surface to the white matter border were counted by using a Neurolucida system (MicroBrightField, Colchester, VT) attached to a Leitz Aristoplan microscope at a final magnification of ×400. Intrarater reliability was evaluated by recounting the same traverses over time and was consistently greater than 95%. Laminar boundaries were determined based on percent values derived from measurements on neighboring Nissl-stained sections. The density of cartridge-like profiles with detectable levels of GAT-1 immunoreactivity (subsequently referred to as cartridge density) for each area of each subject was then obtained by averaging the density values from four traverses.
To verify the cartridge density measures obtained by using the traverse counting technique described above, additional tissue sections from the schizophrenic and normal comparison subjects were processed for GAT immunoreactivity, and labeled cartridges were counted in area 46 by using the fractionator program of the Stereo Investigator system (Version 3.0; MicroBrightField). This program provides unbiased and systematic sampling of counting frames with inclusion and exclusion boundaries (19). For each tissue section, the contour area of layers 2 and 3A was outlined and labeled cartridges were identified in random counting frames, each with an area of 62,500 μm2. Counts were restricted to these layers because of the lamina-specific distribution of labeled cartridges. A minimum of 150 cartridges was counted in each sample except for two schizophrenic subjects (Fig. 2B, see triads 7 and 15). The coefficient of error for cartridge counts was <10% in every subject.
The length of axon cartridges was measured in area 46 in the same locations where cartridge densities were determined. Cartridge lengths were measured by using a ×63 oil-immersion objective lens and the Neurolucida system. For each subject, 50 consecutive cartridges were measured (<25 were sampled in two schizophrenic subjects;Fig. 2B, see triads 7 and 15), and the mean cartridge length for each subject was determined.
Quantification of GAT-1-Immunoreactive Boutons.
Two approaches were used to determine whether the density of all GAT-1-immunoreactive axonal boutons differed across subject groups. First, all sections from the same triad of cases were simultaneously scanned on a AGFA Arcus II scanner, without knowledge of the diagnoses, and the stored images were then displayed on a Micro Computer Imaging Device (Imaging Research, St. Catherine’s, ON, Canada) (14). On each of the images, three rectangles extending from the layer 1–2 border to the layer 6–white matter border and ranging from 100 to 300 μm wide, were drawn within areas 9 and 46 in the same regions where cartridge densities were obtained. Three other rectangles of variable sizes were then placed in the adjacent white matter. Light transmittance values through these boxes were automatically computed, and average values in the gray matter and white matter were determined for each cytoarchitectonic area of each section. Adjusted optical density values were then computed as described (14).
Second, several 5-mm2 portions of cortex were removed from medial area 9 of another set of 40-μm-thick sections from the schizophrenic and normal comparison subjects that had been processed for GAT-1 immunoreactivity. These sections were flat-embedded in Epon 812 and resectioned on an ultramicrotome at 1.0 μm. Serial sections were collected beginning at the tissue–Epon interface. Labeled boutons were then counted on sections 2, 5, and 8 at a final magnification of ×1,000 in random counting boxes (400 μm2) by using the stereo investigator fractionator program. A minimum of 200 labeled boutons was counted in each subject and the coefficient of error for bouton counts was consistently <10%. This same approach was also used to count boutons that were immunoreactive for calretinin.
Statistical Analyses.
Differences between subject groups in demographic characteristics were evaluated with one-way analyses of variance, and measures of cartridge or bouton density were assessed by using analyses of covariance with age, sex, and postmortem interval as covariants, diagnosis as the fixed main effect, and individual cases as a random effect.
RESULTS
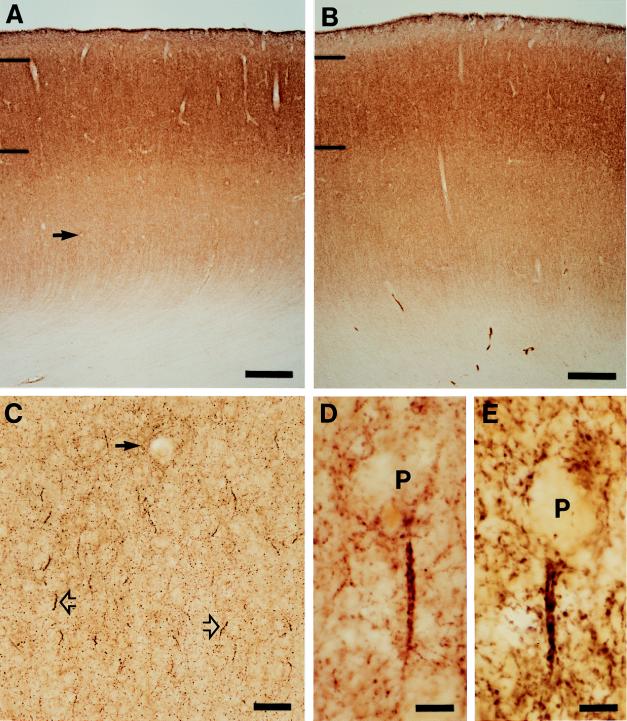
In all three groups of subjects, GAT-1-immunoreactive punctate structures, presumably GABA axon terminals, were distributed across all cortical layers of the PFC but not in the subjacent white matter (Fig. 1 A and B). The intensity of labeling was greater in the superficial than in the deep layers, consistent with the known distribution of GABA terminals (20). Amid this general punctate labeling, distinct parallel arrays of intensely immunoreactive axonal boutons were readily identified below the soma of unlabeled pyramidal cells (Fig. 1 C–E). These structures were morphologically identical to the “axon cartridges” that have previously been shown to represent the terminals of the chandelier class of GABA neurons (10, 21) and to form symmetric synapses on the axon initial segment of pyramidal neurons (21–23). GAT-1-labeled cartridges were distributed across layers 2–6 with the density somewhat lower in layers 3B–5A than in the superficial or deep layers.
Figure 1.
Laminar distribution and overall intensity of GAT-1 immunoreactivity in area 46 is similar in matched normal control (A) and schizophrenic (B) subjects (see Table 1, triad 3). Hash marks indicate the layer 1–2 and 3–4 borders. At higher magnification (C), GAT-1-labeled axon cartridges (open arrows) are readily identified amid the diffuse punctate immunoreactivity. Solid arrow indicates the same blood vessel in A and C. Individual GAT-1-immunoreactive axon cartridges are located below the unlabeled cell bodies of pyramidal neurons (P) in normal control (D) and schizophrenic (E) subjects. [Bars = 500 μm (A and B), 50 μm (C), and 10 μm (D and E).]
In both areas 9 and 46, the traverse counting procedure revealed that the mean density of chandelier axon cartridges in the PFC was significantly decreased by about 40% in the schizophrenic subjects compared with both the normal control and psychiatric subjects (Table 1). Analysis of individual triads of subjects (Fig. 2) revealed that cartridge density was lower in the schizophrenic subject than in both the matched normal control subject and the nonschizophrenic psychiatric subject for 11 of the 15 triads, suggesting that the majority of individuals with schizophrenia exhibit these abnormalities. Furthermore, cartridge density in the schizophrenic subjects was decreased across layers 2–6, although the magnitude of decline was greater in the superficial than in the deep cortical layers. For example, in layers 2 and 3 of both PFC regions, mean cartridge density in the schizophrenic subjects was decreased by 51.6% and 48.4% relative to the normal control and psychiatric subjects, respectively, whereas in layers 5 and 6, mean cartridge density was decreased in the schizophrenic subjects by 39.1% and 34.5%, respectively. However, these laminar differences did not achieve statistical significance.
The decreased relative density of GAT-1-labeled cartridges in the schizophrenic subjects was confirmed by systematic random sampling procedures in layers 2–3A of area 46 in an additional set of tissue sections. Mean (±SD) cartridge density (per mm2) was significantly (P = 0.009) reduced in the schizophrenic subjects (60.2 ± 45.8) compared with normal control subjects (98.0 ± 41.7), and cartridge density was lower in the schizophrenic subject than in the matched control for 12 of the 15 pairs.
Because these findings could have been confounded by a difference in cartridge length between control and schizophrenic subjects, we measured individual cartridges in area 46 from the 12 schizophrenic subjects that showed cartridge density reduction and their matched normal control subjects. However, mean cartridge length in the schizophrenic subjects (17.3 ± 1.5 μm) was not different from that in the control cases (17.9 ± 1.5 μm). In addition, the reduction in cartridge density is not likely to be due an increased PFC volume in the schizophrenic subjects because cortical thickness was decreased in the schizophrenic subjects by 4.2% and 4.9% in areas 46 and 9, respectively, although these differences did not achieve statistical significance. In addition, no significant differences in the thickness of individual cortical layers were found between schizophrenic and control subjects.
Although 13 of the schizophrenic subjects were receiving antipsychotic medications at the time of death, several lines of evidence suggest that this treatment did not account for the decreased cartridge density. (i) The two schizophrenic subjects who had not been on medications for either more than 6 months or 10 years had cartridge densities that were lower than both the matched normal control and psychiatric subjects (see Fig. 2; triads 5 and 7). (ii) Cartridge densities in each of the three psychiatric subjects who had received antipsychotic medications were higher than in the matched schizophrenic subjects in both PFC regions (Fig. 2; triads 3, 10, and 15). (iii) Finally, we examined five additional antipsychotic-treated nonschizophrenic psychiatric subjects and five matched normal controls. Mean cartridge density did not differ between the eight psychiatric subjects treated with antipsychotic agents and the matched normal controls in either area 9 or 46 (Table 2). Thus, it appears unlikely that the observed decrease in cartridge density in schizophrenia is a consequence of treatment with antipsychotic medications.
To determine whether the observed decrease in cartridge density reflected a generalized decrease in GAT-1 immunoreactivity in all GABA terminals, two measures were made of all GAT-1-labeled boutons. (i) Mean (±SD) adjusted optical density measures of GAT-1 immunoreactivity in the same tissue sections used for the cartridge counts did not differ in either area 9 or 46, respectively, among schizophrenic (0.18 ± 0.04; 0.19 ± 0.05), control (0.19 ± 0.04; 0.19 ± 0.04), and psychiatric (0.20 ± 0.04; 0.21 ± 0.05) subjects. Because chandelier neuron axon cartridges constitute only a small proportion of all GABA terminals (11, 12), the absence of any differences in adjusted optical density measures across subject groups suggests that decreased GAT-1 immunoreactivity may be relatively specific to the axon terminals of chandelier neurons. Furthermore, the mean density (per 400 μm2) of all GAT-1-immunoreactive boutons in layers 2–3A of area 9 was not significantly decreased in the schizophrenic subjects (6.6 ± 1.7) compared with the normal controls (6.8 ± 1.6), indicating that the labeled boutons arising from the chandelier class of GABA neurons may be preferentially altered in schizophrenia.
As one additional assessment of the selectivity of the changes for chandelier neuron axon terminals, we also counted the density of calretinin-immunoreactive axonal boutons. This calcium-binding protein, present in about 50% of all GABA neurons in the primate PFC, does not appear to be expressed in the chandelier subclass (18, 24–26). The mean density (per 400 μm2) of all calretinin-labeled boutons in layers 2–3A of area 9 did not differ between the schizophrenic (4.8 ± 1.1) and normal control subjects (4.5 ± 0.8). These findings also suggest that the axon terminals of the chandelier class of PFC GABA neurons are predominately, if not selectively, affected in schizophrenia.
DISCUSSION
The findings of this study suggest that previous reports indicative of altered GABA neurotransmission in the PFC in schizophrenia (6–9) may be a consequence of abnormalities in the chandelier class of GABA-containing local circuit neurons. The decreased density of GAT-1-labeled axon cartridges could be due to a decrease in the number of chandelier neurons. However, this explanation is unlikely because the density, laminar distribution, and size of neurons containing parvalbumin, a calcium-binding protein present in chandelier neurons (21, 27), were not altered in the PFC in the same 15 schizophrenic subjects examined in the present study (28). Consistent with this finding, most other studies have also failed to detect a decrease in the number of PFC neurons in schizophrenia (8, 29, 30). In the one study that did report a decrease in the density of small presumably nonpyramidal neurons in PFC layer 2 (31), the decrease appeared to be associated with the presence of affective traits (32). However, only 2 of the 15 subjects in the present study met criteria for schizoaffective disorder. Thus, the decreased density of axon cartridges observed in the schizophrenic subjects does not appear to be attributable to a diminished number of PFC chandelier neurons in these subjects.
The observed decrease in density of GAT-1-immunoreactive cartridges could represent a reduction in the number of intact cartridges. This could arise as a consequence of abnormalities in the pyramidal neurons that chandelier cartridges innervate. Although further investigations are required to fully address this question, the number of PFC pyramidal neurons does not appear to be decreased in schizophrenia (8, 29–31). In addition, in an investigation that included many of the subjects examined in the present study, no abnormalities were identified in the subpopulation of PFC pyramidal neurons that express nonphosphorylated neurofilament protein (33).
Decreased cartridge density could also be due to a reduction in the number of boutons per cartridge so that some individual cartridges lose their distinctive appearance. Due to the linear arrangement of the boutons within a cartridge, a reduction in bouton number would likely produce shorter cartridges. However, the length of GAT-1-positive cartridges did not differ between schizophrenic and control subjects. Alternatively, our findings might represent a reduction in the number of axon cartridges arising from each chandelier cell, an interpretation consistent with the results of other studies indicating that the number of axon terminals in the PFC are diminished in schizophrenia (14, 29).
In contrast to a change in the actual number of cartridges, the decreased density of GAT-1-labeled cartridges might reflect reduced levels of GAT-1 immunoreactivity in chandelier neuron axon terminals. In fact, during development of the primate PFC, the detectability of other protein markers for chandelier axon cartridges may decline substantially (34) without any apparent loss of terminals (10) or change in the total number of inhibitory synapses (35). Whether due to fewer axon cartridges or decreased GAT-1 levels per cartridge, our findings suggest that inhibition to the axon initial segment of pyramidal cells is altered in the PFC in schizophrenia.
The importance of a decrease in GAT-1-immunoreactive cartridges for our understanding of the pathophysiology of schizophrenia depends, in part, on the selectivity of the findings. Several lines of evidence suggest that the decrease in GAT-1-labeled axon cartridges may be specific to the disease process of schizophrenia. (i) The density of labeled cartridges was not decreased in the matched group of nonschizophrenic psychiatric subjects, indicating that factors such as suicide, chronic psychiatric illness, or comorbid substance abuse or dependence do not explain the findings in the schizophrenic subjects. (ii) The density of GAT-1-immunoreactive cartridges did not differ between schizophrenic subjects with and without a history of alcohol abuse or dependence. (iii) Finally, as indicated above, treatment with antipsychotic medications did not appear to account for the decrease in cartridge density.
Several lines of evidence suggest that alterations in GABA terminals in the PFC in schizophrenia may be relatively selective for those arising from the chandelier neuron class. (i) Although the mean relative density of GAT-1-immunoreactive axon cartridges was reduced by 40% in the schizophrenic subjects, no changes were observed in the total number of GAT-1-labeled axon boutons. Because chandelier neuron axon cartridges constitute less than 1% of all GABA terminals in the cortex (12), a change restricted to chandelier neuron terminals would not be detectable by counting all GABA boutons. However, if all subpopulations of GABA neurons were similarly affected, then such a change would be reflected in the counts of all boutons. (ii) The density of calretinin-immunoreactive boutons did not differ between schizophrenic and control subjects. Calretinin is present in approximately 50% of PFC GABA neurons and is not expressed in chandelier cells in this region (18, 24–26). (iii) Finally, the number of PFC neurons with detectable levels of the mRNA for glutamic acid decarboxylase, the rate-limiting enzyme of GABA synthesis, is reduced in schizophrenia (8), an observation consistent with the notion that alterations in PFC GABA neurotransmission in schizophrenia are restricted to a subset of GABA neurons.
The decreased cartridge density was present across layers 2–6, suggesting that the output activity of multiple populations of PFC pyramidal neurons may be affected in schizophrenia. Such a change would certainly contribute to the substantial alterations in activity within the PFC and in interconnected cortical and subcortical regions (36, 37) that are thought to account for the cognitive abnormalities characteristic of schizophrenia (38, 39). In addition, parvalbumin-containing cortical neurons, which include chandelier cells (21, 27), have been shown to be synaptic targets of dopamine axons (40) and to express dopamine D4 receptors (41). These neurons also receive input from serotonin-containing axons (42). In concert with the results of the present study, these findings raise the possibility that the therapeutic effects of atypical antipsychotic medications with a high affinity for D4 and serotonin 5-HT2 receptors (43) may be mediated, at least in part, via chandelier neuron axon cartridges that are strategically located to regulate pyramidal cell output.
Acknowledgments
We thank Brad Booth and Mary Brady for technical assistance, and Alan Sampson and Claudia Matus for statistical consultation. This work was supported by Public Health Service Grants MH43784, MH45156, and MH00519.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: GABA, γ-aminobutyric acid; GAT, GABA transporter; PFC, prefrontal cortex.
References
- 1.Carpenter W T, Jr, Buchanan R W. N Engl J Med. 1994;330:681–690. doi: 10.1056/NEJM199403103301006. [DOI] [PubMed] [Google Scholar]
- 2.Weinberger D R, Berman K F, Zec R F. Arch Gen Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- 3.Park S, Holzman P S. Arch Gen Psychiatry. 1992;49:975–982. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- 4.Simpson M D C, Slater P, Deakin J F W, Royston M C, Skan W J. Neurosci Lett. 1989;107:211–215. doi: 10.1016/0304-3940(89)90819-7. [DOI] [PubMed] [Google Scholar]
- 5.Reynolds G P, Czudek C, Andrews H B. Biol Psychiatry. 1990;27:1038–1044. doi: 10.1016/0006-3223(90)90039-5. [DOI] [PubMed] [Google Scholar]
- 6.Sherman A D, Davidson A T, Baruah S, Hegwood T S, Waziri R. Neurosci Lett. 1991;121:77–80. doi: 10.1016/0304-3940(91)90653-b. [DOI] [PubMed] [Google Scholar]
- 7.Bird E D, Spokes E G S, Iversen L L. Brain. 1979;102:347–360. doi: 10.1093/brain/102.2.347. [DOI] [PubMed] [Google Scholar]
- 8.Akbarian S, Kim J J, Potkin S G, Hagman J O, Tafazzoli A, Bunney W E, Jr, Jones E G. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 9.Benes F M, Snyder-Marie A, Vincent S, Khan Y. Neuroscience. 1996;75:1021–1031. doi: 10.1016/0306-4522(96)00328-4. [DOI] [PubMed] [Google Scholar]
- 10.Lund J S, Lewis D A. J Comp Neurol. 1993;328:282–312. doi: 10.1002/cne.903280209. [DOI] [PubMed] [Google Scholar]
- 11.Peters A. In: Cerebral Cortex. Jones E G, Peters A, editors. Vol. 1. New York: Plenum; 1984. pp. 361–380. [Google Scholar]
- 12.DeFelipe J, Farinas I. Prog Neurobiol. 1992;39:563–607. doi: 10.1016/0301-0082(92)90015-7. [DOI] [PubMed] [Google Scholar]
- 13.Minelli A, Brecha N C, Karschin C, DeBiasi S, Conti F. J Neurosci. 1995;15:7734–7746. doi: 10.1523/JNEUROSCI.15-11-07734.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glantz L A, Lewis D A. Arch Gen Psychiatry. 1997;54:943–952. doi: 10.1001/archpsyc.1997.01830220065010. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg D R, Lewis D A. J Comp Neurol. 1995;358:383–400. doi: 10.1002/cne.903580306. [DOI] [PubMed] [Google Scholar]
- 16.Daviss S R, Lewis D A. Psychiatry Res. 1995;59:81–96. doi: 10.1016/0165-1781(95)02720-3. [DOI] [PubMed] [Google Scholar]
- 17.Rajkowska G, Goldman-Rakic P S. Cereb Cortex. 1995;5:307–322. doi: 10.1093/cercor/5.4.307. [DOI] [PubMed] [Google Scholar]
- 18.Condé F, Lund J S, Jacobowitz D M, Baimbridge K G, Lewis D A. J Comp Neurol. 1994;341:95–116. doi: 10.1002/cne.903410109. [DOI] [PubMed] [Google Scholar]
- 19.Gundersen H J G, Bagger P, Bendsten T F, Evans S M, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard J R, Pakkenberg B, et al. Acta Pathol Microbiol Immunol Scand. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- 20.Jones E G. Cereb Cortex. 1993;3:361–372. doi: 10.1093/cercor/3.5.361-a. [DOI] [PubMed] [Google Scholar]
- 21.DeFelipe J, Hendry S H C, Jones E G. Proc Natl Acad Sci USA. 1989;86:2093–2097. doi: 10.1073/pnas.86.6.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeFelipe J, Hendry S H C, Jones E G, Schmechel D. J Comp Neurol. 1985;231:364–384. doi: 10.1002/cne.902310307. [DOI] [PubMed] [Google Scholar]
- 23.Somogyi P, Freund T F, Cowey A. Neuroscience. 1982;7:2577–2607. doi: 10.1016/0306-4522(82)90086-0. [DOI] [PubMed] [Google Scholar]
- 24.Gabbott P L A, Bacon S J. J Comp Neurol. 1996;364:609–636. doi: 10.1002/(SICI)1096-9861(19960122)364:4<609::AID-CNE2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 25.Gabbott P L, Jays P R, Bacon S J. J Comp Neurol. 1997;381:389–410. doi: 10.1002/(sici)1096-9861(19970519)381:4<389::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 26.del Rio M R, DeFelipe J. J Neurosci. 1997;17:5143–5154. doi: 10.1523/JNEUROSCI.17-13-05143.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewis D A, Lund J S. J Comp Neurol. 1990;293:599–615. doi: 10.1002/cne.902930406. [DOI] [PubMed] [Google Scholar]
- 28.Woo T-U, Miller J L, Lewis D A. Am J Psychiatry. 1997;154:1013–1015. doi: 10.1176/ajp.154.7.1013. [DOI] [PubMed] [Google Scholar]
- 29.Selemon L D, Rajkowska G, Goldman-Rakic P S. Arch Gen Psychiatry. 1995;52:805–818. doi: 10.1001/archpsyc.1995.03950220015005. [DOI] [PubMed] [Google Scholar]
- 30.Pakkenberg B. Biol Psychiatry. 1993;34:768–772. doi: 10.1016/0006-3223(93)90065-l. [DOI] [PubMed] [Google Scholar]
- 31.Benes F M, McSparren J, Bird E D, SanGiovanni J P, Vincent S L. Arch Gen Psychiatry. 1991;48:996–1001. doi: 10.1001/archpsyc.1991.01810350036005. [DOI] [PubMed] [Google Scholar]
- 32.Vincent S L, Todtenkopf M S, Benes F M. Soc Neurosci Abstr. 1997;23:2199. [Google Scholar]
- 33.Pierri J N, Lewis D A. Soc Neurosci Abstr. 1997;23:2200. [Google Scholar]
- 34.Anderson S A, Classey J D, Condé F, Lund J S, Lewis D A. Neuroscience. 1995;67:7–22. doi: 10.1016/0306-4522(95)00051-j. [DOI] [PubMed] [Google Scholar]
- 35.Bourgeois J-P, Goldman-Rakic P S, Rakic P. Cereb Cortex. 1994;4:78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- 36.Pearlson G D, Petty R G, Ross C A, Tien A Y. Neuropsychopharmacology. 1996;14:1–17. doi: 10.1016/S0893-133X(96)80054-6. [DOI] [PubMed] [Google Scholar]
- 37.Andreasen N C, O’Leary D S, Cizaldo T, Arndt S, Rezai K, Boles Ponto L L, Watkins G L, Hichwa R D. Proc Natl Acad Sci USA. 1996;93:9985–9990. doi: 10.1073/pnas.93.18.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldman-Rakic P S. J Neuropsychiatry. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- 39.Goldberg T E, Gold J M. In: Schizophrenia. Hirsch S R, Weinberger D R, editors. Oxford, U.K.: Blackwell; 1995. pp. 146–162. [Google Scholar]
- 40.Lewis D A, Hawrylak V A, Melchitzky D S, Sesack S R. Soc Neurosci Abstr. 1996;22:1321. [Google Scholar]
- 41.Mrzljak L, Bergson C, Pappy M, Huff R, Levenson R, Goldman-Rakic P S. Nature (London) 1996;381:245–248. doi: 10.1038/381245a0. [DOI] [PubMed] [Google Scholar]
- 42.Jakab R L, Goldman-Rakic P S. Soc Neurosci Abstr. 1996;22:905. [Google Scholar]
- 43.Seeman P, Van Tol H H. Trends Pharmacol Sci. 1994;15:264–270. doi: 10.1016/0165-6147(94)90323-9. [DOI] [PubMed] [Google Scholar]