Abstract
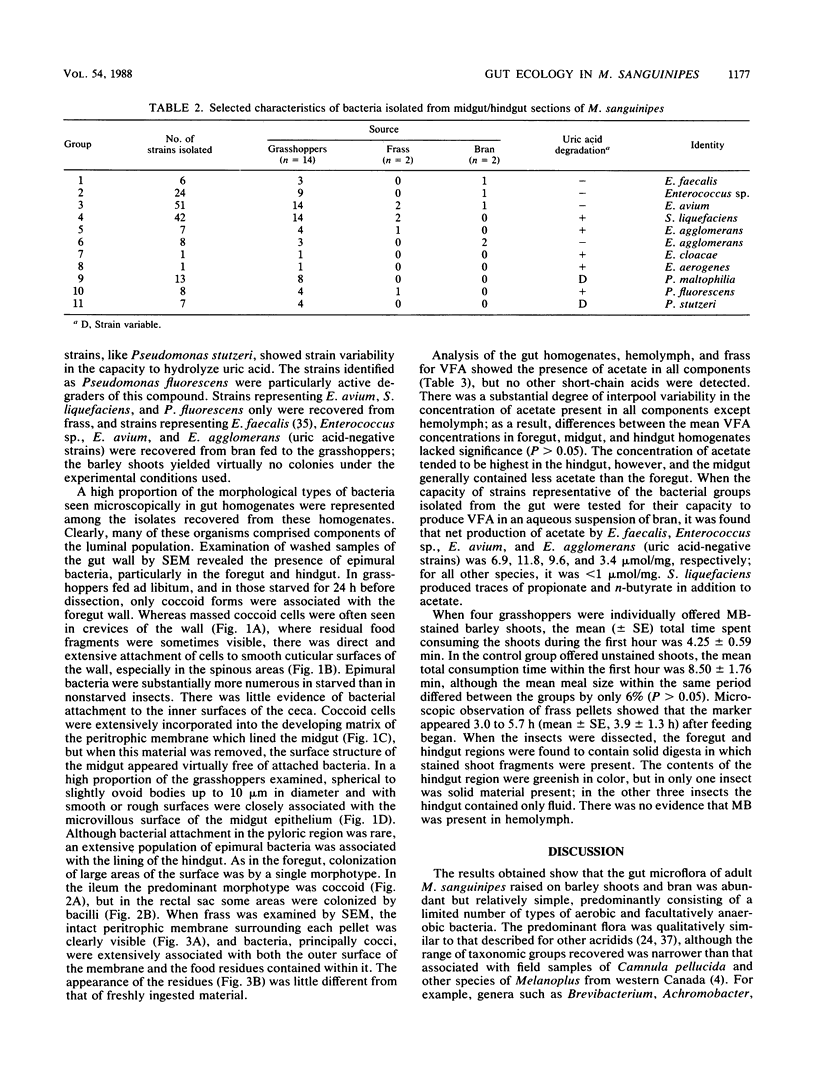
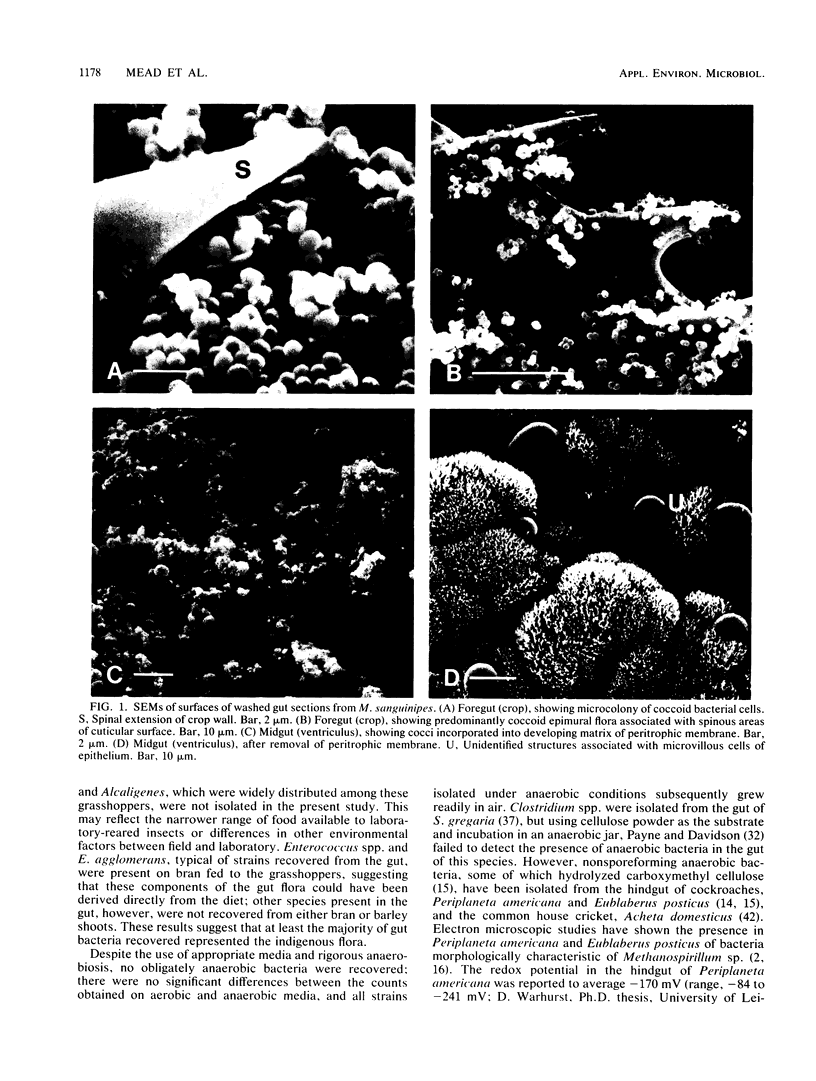
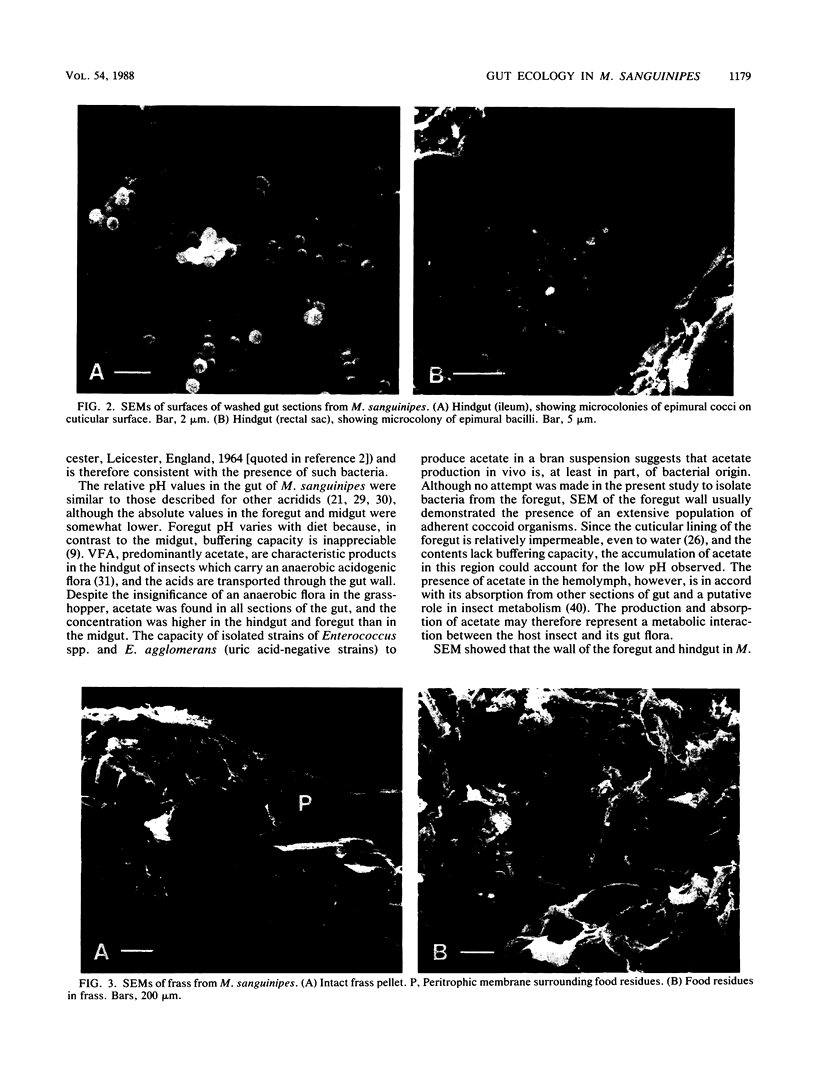
Mean pH values in pooled samples of foregut, midgut, and hindgut from adult Melanoplus sanguinipes, which had been raised in the laboratory on barley shoots and wheat bran, were 5.15, 6.39, and 5.98, respectively. Homogenates of midgut/hindgut sections and frass (feces) yielded colony counts of bacteria by the spread plate method of 5.7 to 5.9 and 5.3 to 5.5 log10 colonies per mg, respectively; there were no significant differences (P > 0.05) between counts obtained on several media or on media incubated aerobically or anaerobically. There was no evidence of significant populations of protozoa, fungi, or obligately anaerobic bacteria associated with the gut. A total of 168 pure strains of bacteria isolated from the gut sections were characterized and assigned to 11 taxonomic groups, including Enterococcus spp., Serratia liquefaciens, Pseudomonas spp., and Enterobacter spp. Numbers of Enterococcus spp. in the gut were 2 to 3 orders of magnitude higher than those of the other genera. Strains representing only four of the groups were recovered from bran fed to the grasshoppers; the barley shoots, which were raised in sterile soil, appeared virtually sterile. Examination of the gut wall by scanning electron microscopy revealed the presence of epimural bacteria in the foregut and hindgut but not in the midgut. The distribution of epimural cocci and bacilli differed with the gut section examined. Numerous spherical to ovoid structures up to 10 μm in diameter, which were not identified, were associated with the microvillous surface of the midgut epithelium. Acetate was present in gut, hemolymph, and frass, and it was shown that representative isolates of Enterococcus spp. and Enterobacter agglomerans produced acetate when incubated in an aqueous suspension of bran. The egestion time of solid digesta, as measured with methylene blue-stained barley shoots, was 3.0 to 5.7 h. The results show that M. sanguinipes supported extensive indigenous populations of luminal and epimural bacteria in the gut which were composed predominantly of facultatively anaerobic species; the relatively short egestion time, indicating rapid passage of digesta through the gut, was consistent with the microscopic appearance of digesta residues in frass and could account, at least in part, for the absence of a significant population of obligately anaerobic bacteria from the gut.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bracke J. W., Cruden D. L., Markovetz A. J. Intestinal microbial flora of the of the American cockroach, Periplaneta americana L. Appl Environ Microbiol. 1979 Nov;38(5):945–955. doi: 10.1128/aem.38.5.945-955.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell D. R., Bryant M. P. Medium without rumen fluid for nonselective enumeration and isolation of rumen bacteria. Appl Microbiol. 1966 Sep;14(5):794–801. doi: 10.1128/am.14.5.794-801.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruden D. L., Markovetz A. J. A thick-walled organism isolated from the cockroach gut by using a spent medium technique. Appl Environ Microbiol. 1980 Jan;39(1):261–264. doi: 10.1128/aem.39.1.261-264.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruden D. L., Markovetz A. J. Carboxymethyl cellulose decomposition by intestinal bacteria of cockroaches. Appl Environ Microbiol. 1979 Sep;38(3):369–372. doi: 10.1128/aem.38.3.369-372.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead L. J., Jones G. A. Isolation and presumptive identification of adherent epithelial bacteria ("epimural" bacteria) from the ovine rumen wall. Appl Environ Microbiol. 1981 Apr;41(4):1020–1028. doi: 10.1128/aem.41.4.1020-1028.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odelson D. A., Breznak J. A. Volatile Fatty Acid production by the hindgut microbiota of xylophagous termites. Appl Environ Microbiol. 1983 May;45(5):1602–1613. doi: 10.1128/aem.45.5.1602-1613.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards A. G., Richards P. A. The peritrophic membranes of insects. Annu Rev Entomol. 1977;22:219–240. doi: 10.1146/annurev.en.22.010177.001251. [DOI] [PubMed] [Google Scholar]
- Stevenson J. P. The normal bacterial flora of the alimentary canal of laboratory stocks of the desert locust, Schistocerca gregaria Forskål. J Invertebr Pathol. 1966 Jun;8(2):205–211. doi: 10.1016/0022-2011(66)90130-3. [DOI] [PubMed] [Google Scholar]
- Tsai C. G., Jones G. A. Isolation and identification of rumen bacteria capable of anaerobic phloroglucinol degradation. Can J Microbiol. 1975 Jun;21(6):794–801. doi: 10.1139/m75-117. [DOI] [PubMed] [Google Scholar]
- Ulrich R. G., Buthala D. A., Klug M. J. Microbiota Associated with the Gastrointestinal Tract of the Common House Cricket, Acheta domestica. Appl Environ Microbiol. 1981 Jan;41(1):246–254. doi: 10.1128/aem.41.1.246-254.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]





