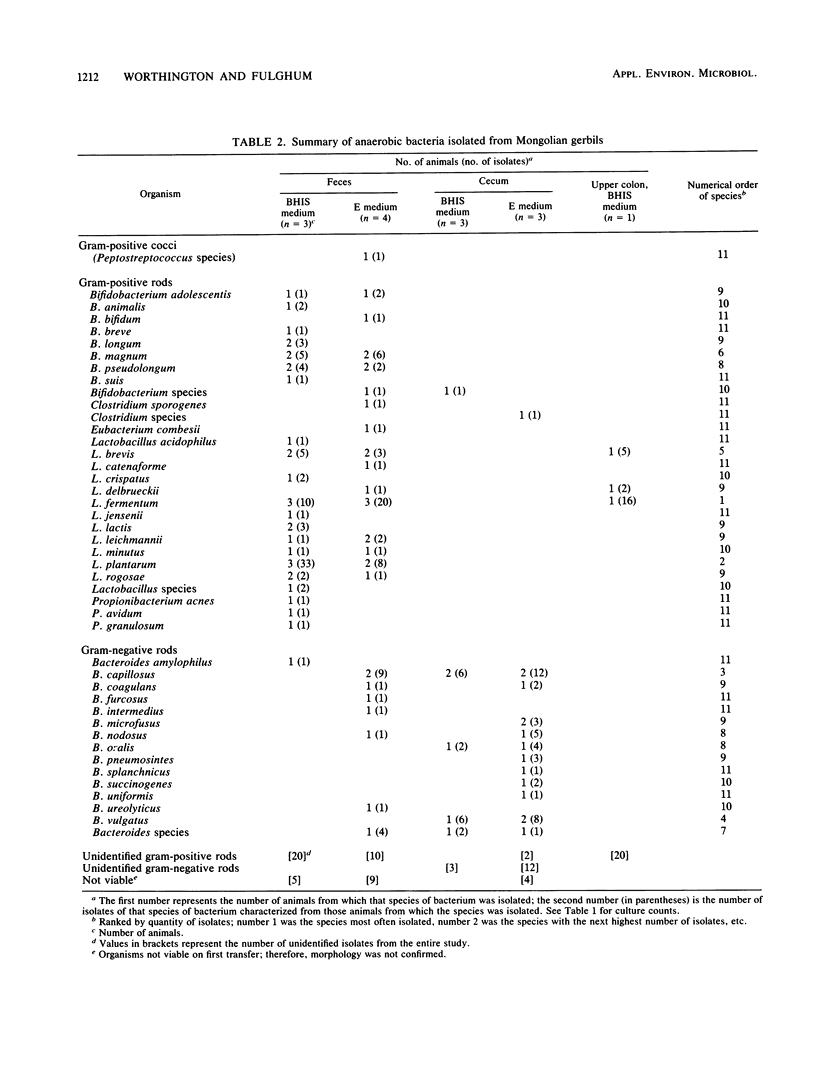
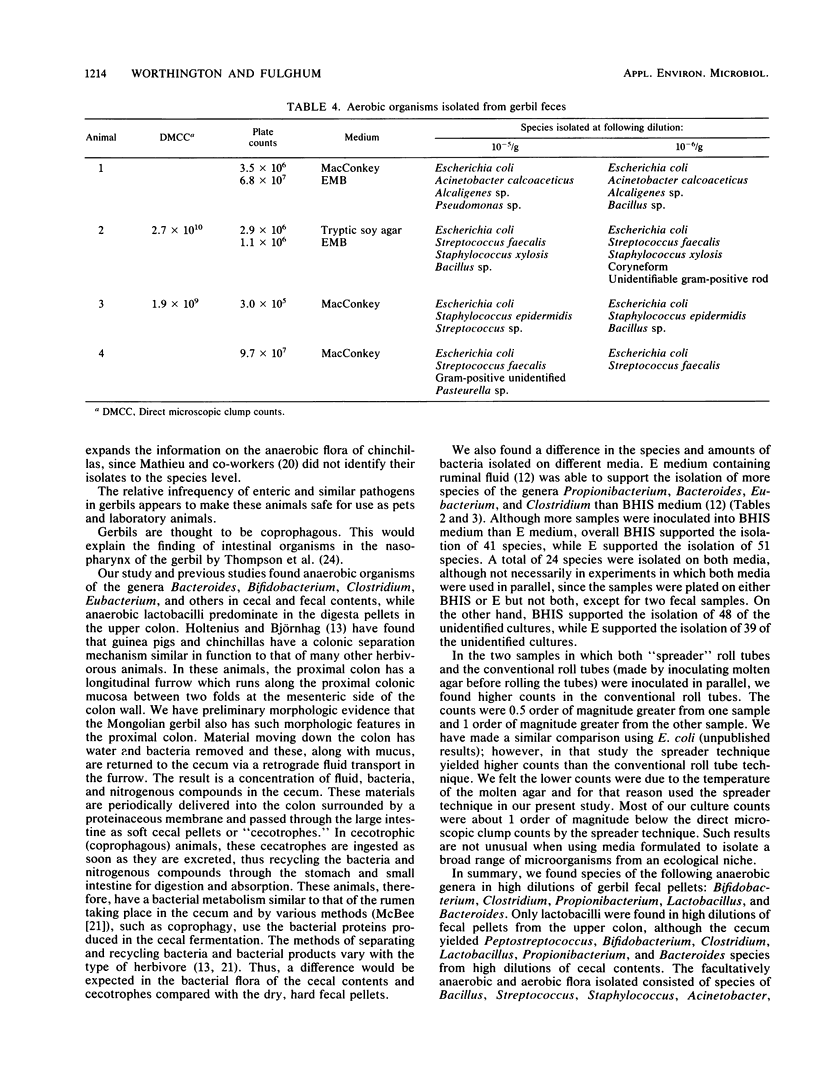
Abstract
The Mongolian gerbil is being increasingly used as a laboratory animal and as a pet. Both chinchillas and gerbils are used as animal models for otitis media and other otic research. Previously, only incomplete information was available regarding the indigenous bacterial flora of the lower intestinal tracts of these coprophagic animals. Using the strict anaerobic methodology of the Virginia Polytechnic Institute Anaerobe Laboratory, we studied the predominant bacterial flora of the cecum and fecal pellets of the gerbil and the chinchilla and the bacterial flora of digesta pellets in the proximal colon. We found species of the following anaerobic genera in high dilutions of gerbil fecal pellets: Bifidobacterium, Clostridium, Propionibacterium, Lactobacillus, and Bacteroides. Only lactobacilli were found in high dilutions of digesta from the upper colon, although the cecum yielded Peptostreptococcus, Bifidobacterium, Clostridium, Lactobacillus, Propionibacterium, and Bacteroides species from high dilutions of cecal contents. The facultatively anaerobic and aerobic flora isolated consisted of species of Bacillus, Streptococcus, Staphylococcus, Acinetobacter, Alcaligenes, Escherichia, Pasteurella, and Pseudomonas plus several unidentifiable organisms. Species of Bifidobacterium, Bacteroides, Eubacterium, and anaerobic Lactobacillus were isolated from chinchillas.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartizal K. F., Wostmann B. S., Wagner M. Distribution and effects of a defined six-member murine-derived microflora in gnotobiotic gerbils. Appl Environ Microbiol. 1984 Apr;47(4):746–751. doi: 10.1128/aem.47.4.746-751.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crociani F., Biavati B., Castagnoli P., Matteuzzi D. Anaerobic ureolytic bacteria from caecal content and soft faeces of rabbit. J Appl Bacteriol. 1984 Aug;57(1):83–88. doi: 10.1111/j.1365-2672.1984.tb02359.x. [DOI] [PubMed] [Google Scholar]
- Fulghum R. S., Brinn J. E., Smith A. M., Daniel H. J., 3rd, Loesche P. J. Experimental otitis media in gerbils and chinchillas with Streptococcus pneumoniae, Haemophilus influenzae, and other aerobic and anaerobic bacteria. Infect Immun. 1982 May;36(2):802–810. doi: 10.1128/iai.36.2.802-810.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulghum R. S., Chole R. A. Bacterial flora in spontaneously occurring aural cholesteatomas in Mongolian gerbils. Infect Immun. 1985 Dec;50(3):678–681. doi: 10.1128/iai.50.3.678-681.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulghum R. S., Chole R. A., Brinn J. E., Branigan A. E. Mongolian gerbil tympanic membrane. Normal and with induced otitis media. Arch Otolaryngol Head Neck Surg. 1987 May;113(5):521–525. doi: 10.1001/archotol.1987.01860050067017. [DOI] [PubMed] [Google Scholar]
- Fulghum R. S., Hoogmoed R. P., Brinn J. E. Longitudinal studies of experimental otitis media with Haemophilus influenzae in the gerbil. Int J Pediatr Otorhinolaryngol. 1985 Jul;9(2):101–114. doi: 10.1016/s0165-5876(85)80010-0. [DOI] [PubMed] [Google Scholar]
- Fulghum R. S., Hoogmoed R. P., Brinn J. E., Smith A. M. Experimental pneumococcal otitis media: longitudinal studies in the gerbil model. Int J Pediatr Otorhinolaryngol. 1985 Oct;10(1):9–20. doi: 10.1016/s0165-5876(85)80052-5. [DOI] [PubMed] [Google Scholar]
- Giebink G. S., Payne E. E., Mills E. L., Juhn S. K., Quie P. G. Experimental otitis media due to Streptococcus pneumoniae: immunopathogenic response in the chinchilla. J Infect Dis. 1976 Dec;134(6):595–604. doi: 10.1093/infdis/134.6.595. [DOI] [PubMed] [Google Scholar]
- Gordon J. H., Dubos R. The anaerobic bacterial flora of the mouse cecum. J Exp Med. 1970 Aug 1;132(2):251–260. doi: 10.1084/jem.132.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M. A., Reddy C. A., Carter G. R. Anaerobic bacteria from the large intestine of mice. Appl Environ Microbiol. 1976 Jun;31(6):907–912. doi: 10.1128/aem.31.6.907-912.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtenius K., Björnhag G. The colonic separation mechanism in the guinea-pig (Cavia porcellus) and the chinchilla (Chinchilla laniger). Comp Biochem Physiol A Comp Physiol. 1985;82(3):537–542. doi: 10.1016/0300-9629(85)90429-3. [DOI] [PubMed] [Google Scholar]
- Lewis D. M., Schram J. L., Meadema S. J., Lim D. J. Experimental otitis media in chinchillas. Ann Otol Rhinol Laryngol Suppl. 1980 May-Jun;89(3 Pt 2):344–350. doi: 10.1177/00034894800890s381. [DOI] [PubMed] [Google Scholar]
- Macy J. M., Farrand J. R., Montgomery L. Cellulolytic and non-cellulolytic bacteria in rat gastrointestinal tracts. Appl Environ Microbiol. 1982 Dec;44(6):1428–1434. doi: 10.1128/aem.44.6.1428-1434.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu X., Durán J. C., Rivas M. Estudio de la flora bacteriana normal de Chinchilla lanigera silvestre. Rev Latinoam Microbiol. 1982 Apr-Jun;24(2):77–82. [PubMed] [Google Scholar]
- Morotomi M., Watanabe T., Suegara N., Kawai Y., Mutai M. Distribution of indigenous bacteria in the digestive tract of conventional and gnotobiotic rats. Infect Immun. 1975 May;11(5):962–968. doi: 10.1128/iai.11.5.962-968.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAEDLER R. W., DUBOS R., COSTELLO R. THE DEVELOPMENT OF THE BACTERIAL FLORA IN THE GASTROINTESTINAL TRACT OF MICE. J Exp Med. 1965 Jul 1;122:59–66. doi: 10.1084/jem.122.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson T. A., Gardner D., Fulghum R. S., Daniel H. J., Allen W. E., Worthington J. M., Williams P. P. Indigenous nasopharyngeal, auditory canal, and middle ear bacterial flora of gerbils: animal model for otitis media. Infect Immun. 1981 Jun;32(3):1113–1118. doi: 10.1128/iai.32.3.1113-1118.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veilleux B. G., Rowland I. Simulation of the rat intestinal ecosystem using a two-stage continuous culture system. J Gen Microbiol. 1981 Mar;123(1):103–115. doi: 10.1099/00221287-123-1-103. [DOI] [PubMed] [Google Scholar]


