Abstract
Single nerve fiber responses to NaCl and their inhibition by amiloride were compared among the chorda tympani (CT) and glossopharyngeal (IXth), and their cross-regenerated nerves in the C57BL/KsJ mice. The CT nerve innervating the anterior part of the tongue contained approximately equal numbers of two types of NaCl-responsive neurons; one type showed strong suppression of NaCl responses by amiloride [amiloride-sensitive (AS) type], and the other type showed only weak or no suppression of NaCl responses by amiloride [amiloride-insensitive (AI) type]. In contrast, the IXth nerve innervating the posterior part of the tongue has almost exclusively the AI type. This relative abundance of the AS and AI types of fibers was not altered by cross-regeneration of the two gustatory nerves into the reverse tongue regions. This suggests that regenerated taste axons selectively recouple with the appropriate type of receptor cell whether they innervate the front or the back of the tongue. Such selective synapse reformation may help explain the stability of response profiles of taste neurons during continual receptor cell turnover.
Taste receptor cells turn over with an average life span of about 10 days in mammals (1–3). The replacement of receptor cells is accompanied by continuing synaptic reconnection between newly formed taste cells and first-order gustatory fibers. Little is known about how a stable sensory code for taste quality is maintained under such continual synaptic reconnection. More than 20 years ago it was observed (4) that branches of a single taste fiber have very similar relative responsiveness to taste stimuli, implying that a given class of taste axons maintains a persistent association with its corresponding class of receptor cells. However, since then little additional evidence for this possibility has been reported. This may largely be because of the lack of a selective taste modifier to identify particular taste receptor types that taste axons innervate.
Recently, it was found in many mammals that a sodium transport blocker, amiloride, specifically inhibits taste responses to NaCl, but does not suppress responses to sweet, sour, and bitter substances (5–7). Using this specific NaCl response inhibitor, subsequent neural response analyses in rodents suggested the existence of two subtypes of receptor mechanisms for NaCl: amiloride-sensitive and amiloride-insensitive. These two subtypes were localized in taste buds in fungiform papillae located on the anterior two-thirds of the tongue, which are innervated by the chorda tympani (CT) nerve (8–10). In marked contrast, it was also reported that in C57BL/KsJ mice (11) and SHR and WKY rats (12) the taste buds in vallate and foliate papillae located on the posterior one-third of the tongue, innervated by the glossopharyngeal (IXth) nerve, may lack the amiloride-sensitive subtype.
It has been shown that transection of the gustatory nerves (the CT and IXth nerves) results in degeneration of taste buds within 7 days, and the taste buds reappear after regeneration of the peripheral nerve fibers into the taste papillae. Only epithelium that normally expresses taste buds (e.g., dorsal tongue) and chemosensory nerves are competent to support the formation and maintenance of taste buds (13–15). In the present study, we made cross-union anastomoses between the CT and IXth nerves of C57BL/KsJ mice similar to those previously performed in rats (16), and we investigated the persistence of specific synaptic connections between amiloride-sensitive and amiloride-insensitive taste axons and cells after cross-regeneration.
MATERIALS AND METHODS
Animals.
Subjects were adult male and female mice of C57BL/KsJ strain (8–25 weeks of age), ranging in weight from 20 to 32 g. At 8–10 weeks of age, mice were divided into six groups with two intact control groups (Intact CT, 8; Intact IX, 8) and the following four experimental groups with differing unilateral end-to-end sensory nerve anastomoses.
Cross-Union Anastomoses of Taste Nerves.
The operative methods were the same as those previously employed in rats (16) (Fig. 1A). (i) CTc-CTp (regeneration control, n = 10): the CT nerve was cut and the central portion of the CT was rejoined to the peripheral portion. (ii) IXc-IXp (regeneration control, n = 10): the IXth nerve was cut and the central portion of the IXth was rejoined to the peripheral portion. (iii) CTc-IXp (cross-regenerated CT, n = 15): the central portion of the CT nerve was sutured to the peripheral part of the IXth nerve. (iv) IXc-CTp (cross-regenerated IX, n = 15): the central part of the IXth nerve was joined to the peripheral part of the CT nerve.
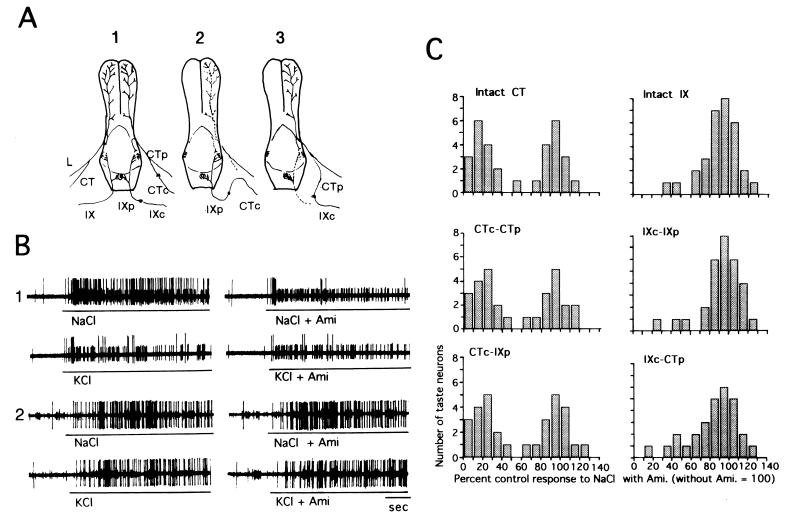
Figure 1.
(A) Schematic drawing of taste nerve crosses and normal taste nerve innervation of the mouse tongue. The normal innervation is shown on the left side of the tongue in A-1. The glossopharyngeal nerve (IX) innervates taste buds in the circumvallate and foliate papillae. The chorda tympani (CT) nerve joins the lingual nerve (L) before entering the tongue and distributes to the anterior two-thirds of the tongue, including a small contribution to the foliate papillae. On the right side in A-1, the CT nerve was cut and the central portion of the CT was rejoined to the peripheral portion of the CT (CTc-CTp). The IXth nerve was cut and the central portion of the IXth was rejoined to the peripheral portion of the Ixth (IXc-IXp). In A-2, the central portion of the CT nerve was sutured to the peripheral part of the IXth nerve (CTc-IXp). In A-3, the central part of the IXth nerve was joined to the peripheral part of the CT nerve (IXc-CTp). (B) Sample recordings of single-fiber responses of the CT nerve (1) and IXth nerve (2) to 0.1 M NaCl and 0.1 M KCl with and without 0.1 mM amiloride. There are two CT units, one large and one small. The larger one showed amiloride inhibition of NaCl responses [amiloride-sensitive (AS) type], but the smaller one did not [amiloride-insensitive (AI) type]. The IX unit was an AI fiber. (C) Distributions of taste neurons with their percentage of control responses to 0.1 M NaCl with 0.1 mM amiloride (control responses to NaCl without amiloride = 100%) in the intact CT (n = 31), intact IX (n = 31), CTc-CTp (n = 29), IXc-IXp (n = 30), CTc-IXp (n = 31), and IXc-CTp (n = 29). In the intact control groups, mean responses (±SD) to NaCl without and with amiloride were 73.4 ± 45.9 and 33.5 ± 23.7 impulses/10 s, respectively, for the CT fibers (mean percent control response, 55.6 ± 38.2%) and 43.6 ± 20.6 and 37.4 ± 16.7 impulses/10 s, respectively, for the IXth fibers (mean percent control response, 89.5 ± 20.2%). There were two groups of taste fibers in the distribution (bimodal in shape, amiloride-sensitive and amiloride-insensitive types) for the intact, regenerated, and cross-regenerated CT nerve, whereas most fibers of the intact, regenerated, and cross-regenerated IXth nerve were amiloride-insensitive, thereby producing unimodal distributions.
Recordings of Responses from Intact and Regenerated Taste Nerves.
After 13–18 weeks these mice were reoperated under pentobarbital anesthesia to expose the regenerated nerve and dissect single or a few fibers of the nerve for electrophysiological recording. The procedures of dissection and recording of responses of the regenerated nerve fibers were the same as those previously used for the nonoperated normal CT and IXth nerve fibers of intact animals (9, 11, 17). Briefly, under pentobarbital anesthesia the trachea of each animal was cannulated, and the mouse was then fixed in the supine position with a head holder to allow dissection of the CT or IXth nerve. The hypoglossal nerve was transected bilaterally to prevent tongue movements. The right CT nerve was exposed at its exit from the lingual nerve by removal of internal pterygoid muscle. The CT nerve was then dissected free from surrounding tissues and cut at the point of its entry to the bulla. The right IXth nerve was exposed by removal of the digastricus muscle and posterior horn of the hyoid bone. The IXth nerve was then dissected free from underlying tissues and cut near its entrance to the posterior lacerated foramen. Single or a few fibers of the nerve were teased apart with a pair of needles and lifted on a silver wire electrode. An indifferent electrode was placed in nearby tissue. Impulse discharges resulting from chemical stimulations of the tongue were fed into an amplifier (Iyodenshikogaku K-1, Nagoya, Japan), monitored on an oscilloscope and audiomonitor, recorded on a recorder (Nihon-kohden, WS-641G, Tokyo), and stored on magnetic tape for later analysis. For chemical stimulation of the fungiform taste papillae, the anterior one-half of the mouse’s tongue was enclosed in a flow chamber. For chemical stimulation of the vallate and foliate papillae, an incision was made on each side of the animal’s face from the corner of the mouth to just above the angle of the jaw, and the papillae were exposed and their trenches opened via slight tension applied through a small suture sewn in the tip of the tongue. Solutions were delivered to each part of the tongue (into the chamber for the anterior part) by gravity flow and flowed over the tongue for a controlled period. Electrophysiological recording from each regenerated nerve began with an examination of multiunit activity to mechanical (pressing and stretching the tongue by a plastic forceps with a tip diameter of about 2.0 mm) and chemical (0.1–0.3 M NaCl and 0.1 M NH4Cl) stimulation. Of 50 operated mice, 32 (CTc-CTp, 8; IXc-IXp, 8; CTc-IXp, 8; IXc-CTp, 8) showed good multiunit responses of their regenerated nerve to chemical stimuli and were used for further single-fiber experiments.
Chemical Stimuli.
Chemical solutions used for single-fiber recording were 0.01–1.0 M NaCl, 0.1 M KCl, 0.1 M NH4Cl, and 0.01 M HCl, and mixtures of 0.01–1.0 M NaCl and 0.1 mM amiloride HCl. These chemicals were dissolved in distilled water at ≈24°C. The order of chemical stimulation basically was fixed as 0.1 M NaCl, 0.1 M KCl, 0.1 M NH4Cl, 0.01 M HCl, and 0.01, 0.03, 0.1, 0.3, and 1.0 M NaCl. Then the NaCl solutions with 0.1 mM amiloride were tested. After the series of stimulations with amiloride, the 0.01–1.0 M NaCl solutions without amiloride were repeatedly applied to check the recovery after amiloride inhibition. In most cases, after confirming the recovery (more than 85% of control levels of response frequencies, monitored by a spike counter), these series of stimulations were repeated. During chemical stimulation of the tongue, the test solution flowed for ≈20 s at the same flow rate as the distilled water used for rinsing the tongue (≈0.5 ml/s). The tongue was rinsed during the interval of ≈1 min between successive stimulations. To examine amiloride inhibition of NaCl responses, the tongue was treated with amiloride for 1 min by running amiloride over the tongue before the stimulation with the mixture of NaCl and amiloride.
Data Analysis.
Single fibers were identified by uniform spike height, singular wave form, and examination of latencies between contiguous spikes. Frequency–time histograms of impulse discharges before, during, and after chemical stimulation of the tongue were made by means of a spike-analysis system (Iyodenshikogaku, SAS-1). For data analysis, I used the net average frequency for the first 10 s after the stimulus onset obtained by subtracting the spontaneous frequency for the 10-s period before stimulation.
RESULTS
In control mice there were approximately equal numbers of two types of NaCl-responsive CT fibers. One type showed strong suppression of NaCl responses by amiloride [amiloride-sensitive (AS) type; Fig. 1B, large unit), and the other type showed only weak or no suppression of NaCl responses by amiloride [amiloride-insensitive (AI) type; Fig. 1B, small unit]. Consequently, when the percentage of inhibition by amiloride is plotted for each neuron, a bimodal distribution results (control = 100) (Fig. 1C, Intact CT). This suggests the existence of both AS and AI receptor components for NaCl in taste cells on the anterior tongue. Most IXth fibers in control mice were amiloride-insensitive, and their distribution thus was unimodal (Fig. 1C, Intact IX). This indicates that AS receptor components for NaCl are predominantly located at the front of the tongue, as suggested by the previous multifiber response study (11). Control regeneration of a nerve into its normal tongue region did not significantly affect the distributions of amiloride sensitivities in the CT and IXth populations (CTc-CTp and IXc-IXp), demonstrating the reproducibility of amiloride sensitivities of regenerated taste cells. Importantly, cross-regeneration of a nerve into a different tongue region produced little or no alteration in bimodal and unimodal frequency distributions of amiloride sensitivities in the cross-regenerated CT and IXth populations, respectively (CTc-IXp and IXc-CTp). This fixed abundance of fiber types suggests that the cross-regenerated CT fibers preferentially synapse with (or induce) AS taste cells in the posterior tongue region, whereas IXth axons preferentially contact AI taste cells in the anterior tongue region.
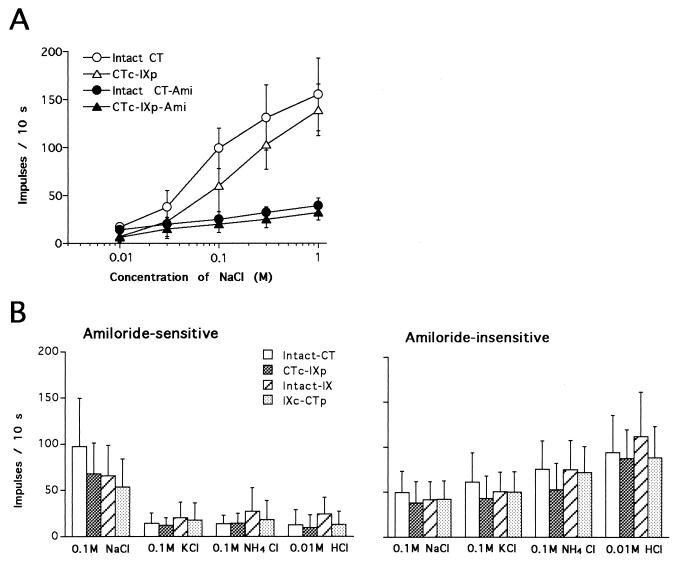
Sensitivities to amiloride and NaCl on the anterior and posterior tongue regions innervated by control and cross-regenerated CT nerves were not greatly different [ANOVA for concentration responses to NaCl between the two groups in Fig. 2A: F(1, 13) = 3.97, P > 0.05 for NaCl responses before amiloride; F(1, 13) = 3.35, P > 0.05 for those after; ANOVA for concentration response to amiloride between the two groups in Fig. 2B: F(1, 8) = 0.55, P > 0.05]. Only the threshold and the Kd value for NaCl responses were slightly higher on the posterior than on the anterior region. [Responses to 0.01 M NaCl were significantly larger in the intact CT compared with the CTc-IXp (t test, P < 0.05). The Kd values: ≈0.09 M for intact CT and ≈0.11 M for CTc-IXp]. This may be because of the recessed location of taste buds in the circumvallate trench or the effects of saliva from the von Ebner’s gland located beneath the circumvallate papilla (18). There also was no prominent difference in response profiles and frequencies of AS and AI fibers in control and cross-regenerated populations [Fig. 2C: ANOVA among four groups, F(3, 30) = 0.66, P > 0.05 for AS fibers; F(3, 71) = 1.14, P > 0.05 for AI fibers]. As previously suggested in rats (8) and hamsters (10), the AS type of taste neurons was narrowly responsive to NaCl (labeled N-type), whereas AI neurons were broadly responsive to various electrolytes (labeled H or E type).
Figure 2.
(A) Concentration-response relationships for NaCl with and without 0.1 mM amiloride in the amiloride-sensitive type of taste neurons of control (Intact CT, n = 8) and cross-regenerated CT (CTc-IXp, n = 7). Vertical bars indicate SDs. (B) Response profiles to 0.1 M NaCl, 0.1 M KCl, 0.1 M NH4Cl, and 0.01 M HCl in the amiloride-sensitive type (showing less than 50% of control NaCl responses after amiloride) of taste neurons of control CT (Intact CT, n = 14), cross-regenerated CT (CTc-IXp, n = 14), control IX (Intact IX, n = 2), and cross-regenerated IX (IXc-CTp, n = 4), and in the amiloride-insensitive type (showing more than 60% of control NaCl responses after amiloride) of taste neurons of control CT (Intact CT, n = 14), cross-regenerated CT (CTc-IXp, n = 14), control IX (Intact IX, n = 25), and cross-regenerated IX (IXc-CTp, n = 22). Vertical bars indicate SDs.
DISCUSSION
The present study reveals that the CT nerve innervating the anterior part of the tongue contained two types of NaCl-responsive neurons, one synapsing with AS receptor cells (AS or N type), and the other with AI cells (AI, H, or E type). In contrast, the IXth nerve innervating the posterior part of the tongue almost exclusively has the AI type (>90%). The relative abundance of the two types of fibers was not altered by cross-regeneration of the two gustatory nerves into the reverse tongue regions. A strong influence of taste axons on taste cells has been suggested for induction of taste buds during a sensitive period in development in rats (19), for maintenance of adult taste buds trophically in various mammals (20), and for regulation of taste cell expression of the neural cell adhesion molecule in rats (21). However, the results of the present study do not require that cross-regenerated AS-type CT fibers induce and/or expand the population of AS receptor cells in the posterior tongue. Taste neuron induction of AS receptor cells may not be necessary because immunohistochemically identified AS channels are present in taste cells of fungiform taste buds days or weeks before robust N type fiber discharges arise (22). As shown in Fig. 1C Top Right, in the control IXth populations 5–10% of the fibers were amiloride-sensitive. Because there are about four times as many taste buds in the posterior than the anterior region (23, 24), there may be enough posterior AS taste cells to accommodate all cross-regenerating CT AS fibers.
In contrast to the present results, previous cross-reinnervation studies on the rat whole nerve activities have demonstrated that relative taste-response profiles of cross-regenerated gustatory nerves [crossed between the CT and IXth (16) or greater superficial petrosal nerves (25)] appeared to be influenced by their new peripheral gustatory receptor populations, suggesting tissue-specific taste nerve responses. Tissue-specific whole-nerve responses would occur in a case in which a limited set of foreign axons was able to diverge as it encountered many receptor cells of the matching type (26). Such increased axon divergence should increase the firing frequencies of individual axons. Yet, the discharge rate failed to increase in the CT AI fibers sent into the posterior tongue region, even though the great abundance of IXth AI fibers implies a corresponding abundance of posterior AI receptor cells. This absence of increased firing rates suggests either that there was little functional divergence of CT AI fibers onto the many AI receptors in the posterior tongue or that the foliate and vallate trenches slowed the rise time of NaCl application thereby preventing brisk responses. Therefore, flexible branching of taste axons relative to the abundance of their matching receptor cells would favor tissue-specific responses, whereas limited size of branching of taste axons would have favored the mouse nerve-specific AS and AI responses. Because there are, however, many other possibilities, future studies are needed to clarify this issue.
It has been shown in rats that amiloride sensitivity of the taste receptor system varies as a function of various environmental and hormonal influences, such as deprivation of dietary sodium (27) and administration of aldosterone (28). In the present study possible influences by desalivation of ipsilateral parotid, sublingual, and submaxillary glands during degeneration and regeneration of the CT and IXth nerves might also be involved in the observed differences in amiloride sensitivities of the mouse-regenerated nerves. However, the relative abundance of the AS and AI types of fibers (Fig. 1C) and mean relative frequencies (data not shown) to taste stimuli tested in the regeneration control group were not different from those in intact control mice. Therefore, influences by such environmental factors may be negligible in this study. In the case of reduction of NaCl responses of the rat CT nerve by sodium-deficient diet, significant reduction of the responses was found only in N type fibers (29). This suggests that even though such environmental factors may alter the number of sodium channels of taste cells and reduce the taste sensitivity, they may not affect connectivity between particular taste axons and cells.
In conclusion, the present findings are consistent with the view that AS axons selectively innervate AS taste progenitor cells. Probably, selective innervation also applies to AI axons and their matching AI progenitor cells. Synapse formation between matched sets of taste axons and cells could contribute to the development and maintenance of particular receptor components in the taste cells. Selective synapse formation could explain the stability of response profiles of particular classes of taste axons, thereby maintaining stable sensory signals for taste quality in the presence of continual receptor cell turnover.
Acknowledgments
I thank Drs. Bruce Oakley (University of Michigan), Gary K. Beauchamp (Monell Chemical Senses Center), David V. Smith (University of Maryland), and Yasushi Fukami (Asahi University) for critical reading of and valuable comments on the manuscript. This study is supported in part by Grant-in-Aid Nos. 09470407 and 09557147 for Scientific Research from the Ministry of Education, Science, and Culture of Japan.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: CT, chorda tympani; IXth, glossopharyngeal; AS, amiloride sensitive; AI, amiloride insensitive.
References
- 1.Beidler L M, Smallman R L. J Cell Biol. 1965;27:263–272. doi: 10.1083/jcb.27.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farbman A I. Cell Tissue Kinet. 1980;13:349–357. doi: 10.1111/j.1365-2184.1980.tb00474.x. [DOI] [PubMed] [Google Scholar]
- 3.Tateda M, Shishido Y, Kitao K, Suzuki Y. Arch Histol Jpn. 1981;44:485–495. doi: 10.1679/aohc1950.44.485. [DOI] [PubMed] [Google Scholar]
- 4.Oakley B. Chem Sens Flav. 1975;2:52–63. [Google Scholar]
- 5.Heck G L, Mierson S, DeSimone J A. Science. 1984;223:403–405. doi: 10.1126/science.6691151. [DOI] [PubMed] [Google Scholar]
- 6.Hellekant G, DuBois G E, Robert T W, van der Wel H. Chem Senses. 1988;13:89–93. [Google Scholar]
- 7.Schiffman S S, Lockhead E, Maes F W. Proc Natl Acad Sci USA. 1983;80:6136–6140. doi: 10.1073/pnas.80.19.6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ninomiya Y, Funakoshi M. Brain Res. 1988;445:319–325. doi: 10.1016/0006-8993(88)90777-9. [DOI] [PubMed] [Google Scholar]
- 9.Ninomiya Y, Sako N, Funakoshi M. J Comp Physiol. 1989;166:1–5. doi: 10.1007/BF00190204. [DOI] [PubMed] [Google Scholar]
- 10.Hettinger T P, Frank M E. Brain Res. 1990;513:24–34. doi: 10.1016/0006-8993(90)91085-u. [DOI] [PubMed] [Google Scholar]
- 11.Ninomiya Y, Tanimukai T, Yoshida S, Funakoshi M. Physiol Behav. 1991;49:913–918. doi: 10.1016/0031-9384(91)90203-z. [DOI] [PubMed] [Google Scholar]
- 12.Formaker B K, Hill D L. Physiol Behav. 1991;50:765–769. doi: 10.1016/0031-9384(91)90015-g. [DOI] [PubMed] [Google Scholar]
- 13.Guth L. Anat Rec. 1958;130:25–37. doi: 10.1002/ar.1091300104. [DOI] [PubMed] [Google Scholar]
- 14.Zalewski A A. J Comp Neurol. 1981;200:309–314. doi: 10.1002/cne.902000302. [DOI] [PubMed] [Google Scholar]
- 15.Farbman A I. J Embryol Exp Morphol. 1969;22:55–68. [PubMed] [Google Scholar]
- 16.Oakley B. J Physiol. 1967;188:353–371. doi: 10.1113/jphysiol.1967.sp008143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ninomiya Y. J Neurophysiol. 1997;76:3550–3554. doi: 10.1152/jn.1996.76.5.3550. [DOI] [PubMed] [Google Scholar]
- 18.Gurkan S, Bradley R M. Chem Senses. 1988;13:655–661. [Google Scholar]
- 19.Hosley M A, Hughes S E, Morton L L, Oakley B. J Neurosci. 1987;7:2075–2080. doi: 10.1523/JNEUROSCI.07-07-02075.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oakley B. In: Olfactory and Gustatory Influences on the Central Nervous System. Pfaff D, editor. New York: The Rockefeller Univ. Press; 1985. pp. 92–103. [Google Scholar]
- 21.Smith D V, Klevisky R, Akeson R A, Shipley M T. J Comp Neurol. 1994;347:187–196. doi: 10.1002/cne.903470204. [DOI] [PubMed] [Google Scholar]
- 22.Stewart R E, Hill D L. In: Mechanisms of Taste Transduction. Simon S A, Roper S D, editors. Boca Raton, FL: CRC; 1993. pp. 127–160. [Google Scholar]
- 23.Miller I J. In: Food Intake and Chemical Senses. Katsuki Y, Sato M, Takagi S F, Oomura Y, editors. Tokyo: Tokyo Univ. Press; 1977. pp. 679–684. [Google Scholar]
- 24.Miller I J, Smith D V. Physiol Behav. 1984;32:275–285. doi: 10.1016/0031-9384(84)90142-2. [DOI] [PubMed] [Google Scholar]
- 25.Nejad M S, Beidler L M. Ann NY Acad Sci. 1987;510:523–526. [Google Scholar]
- 26.Oakley B. In: Olfaction and Taste X. Doving K B, editor. Oslo: GCS Press; 1990. pp. 186–195. [Google Scholar]
- 27.Hill D L, Phillips L M. J Neurosci. 1994;14:2904–2910. doi: 10.1523/JNEUROSCI.14-05-02904.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herness M S. Comp Biochem Physiol. 1992;103A:269–273. doi: 10.1016/0300-9629(92)90578-e. [DOI] [PubMed] [Google Scholar]
- 29.Contreras R J, Frank M. J Gen Physiol. 1979;73:569–594. doi: 10.1085/jgp.73.5.569. [DOI] [PMC free article] [PubMed] [Google Scholar]