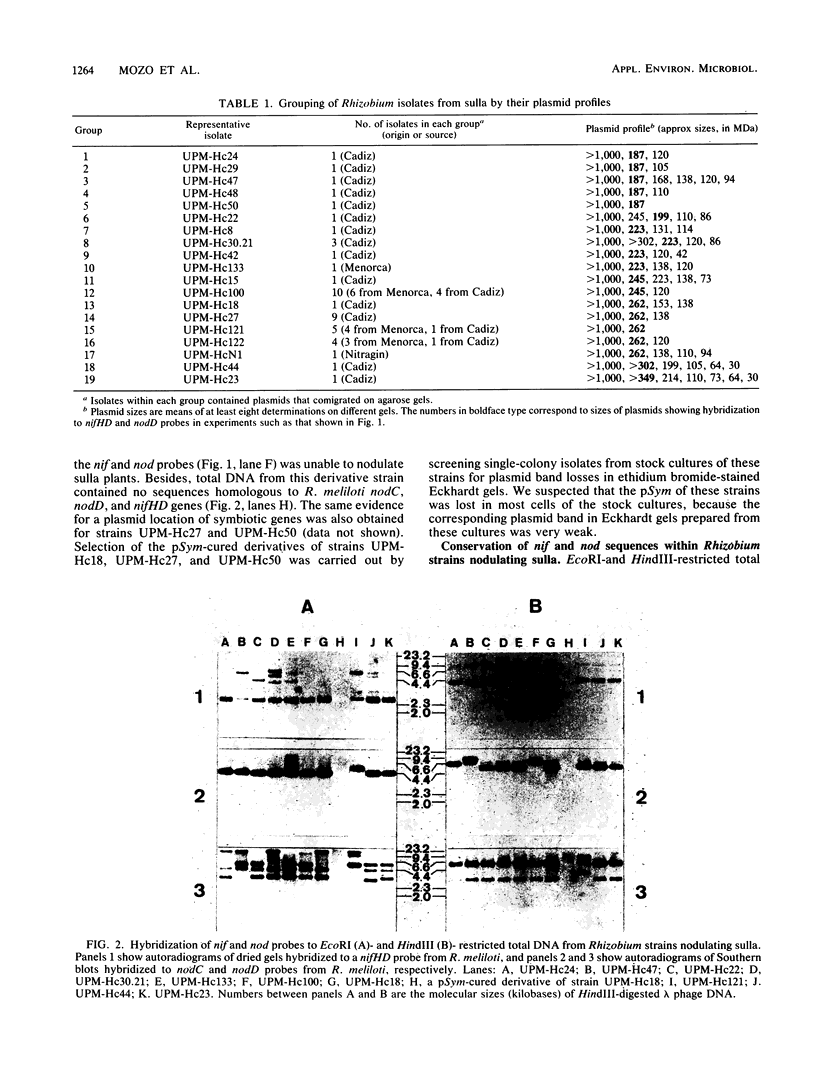
Abstract
Forty-five Rhizobium strains nodulating sulla (Hedysarum coronarium L.), isolated from plants grown in different sites in Menorca Island and southern Spain, were examined for plasmid content and the location and organization of nif (nitrogen fixation) and nod (nodulation) sequences. A great diversity in both number and size of the plasmids was observed in this native population of strains, which could be distributed among 19 different groups according to their plasmid profiles. No correlation was found between plasmid profile and geographical origin of the strains. In each strain a single plasmid ranging from 187 to 349 megadaltons hybridized to Rhizobium meliloti nifHD and nodD DNA, and in three strains the spontaneous loss of this plasmid resulted in the loss of the nodulation capacity. In addition to the symbiotic plasmid, 18 different cryptic plasmids were identified. A characteristic cryptic plasmid of >1,000 megadaltons was present in all strains. Total DNA hybridization experiments, with nifHD and portions of nodC and nodD genes (coding for common nodulation functions) from R. meliloti as probes, demonstrated that both the sequence and organization of nif and common nod genes were highly conserved within rhizobia nodulating sulla. Evidence for reiteration of nodD sequences and for linkage of nodC to at least one copy of nodD was obtained for all the strains examined. From these results we conclude that Rhizobium strains nodulating sulla are a homogeneous group of symbiotic bacteria that are closely related to the classical fast-growing group of rhizobia.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beringer J. E. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974 Sep;84(1):188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkardt B., Burkardt H. J. Visualization and exact molecular weight determination of a Rhizobium meliloti megaplasmid. J Mol Biol. 1984 May 15;175(2):213–218. doi: 10.1016/0022-2836(84)90475-3. [DOI] [PubMed] [Google Scholar]
- Bánfalvi Z., Sakanyan V., Koncz C., Kiss A., Dusha I., Kondorosi A. Location of nodulation and nitrogen fixation genes on a high molecular weight plasmid of R. meliloti. Mol Gen Genet. 1981;184(2):318–325. doi: 10.1007/BF00272925. [DOI] [PubMed] [Google Scholar]
- Chua K. Y., Pankhurst C. E., Macdonald P. E., Hopcroft D. H., Jarvis B. D., Scott D. B. Isolation and characterization of transposon Tn5-induced symbiotic mutants of Rhizobium loti. J Bacteriol. 1985 Apr;162(1):335–343. doi: 10.1128/jb.162.1.335-343.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin D., Ditta G., Helinski D. R. Clustering of nitrogen fixation (nif) genes in Rhizobium meliloti. J Bacteriol. 1982 Jan;149(1):221–228. doi: 10.1128/jb.149.1.221-228.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Eckhardt T. A rapid method for the identification of plasmid desoxyribonucleic acid in bacteria. Plasmid. 1978 Sep;1(4):584–588. doi: 10.1016/0147-619x(78)90016-1. [DOI] [PubMed] [Google Scholar]
- Göttfert M., Horvath B., Kondorosi E., Putnoky P., Rodriguez-Quiñones F., Kondorosi A. At least two nodD genes are necessary for efficient nodulation of alfalfa by Rhizobium meliloti. J Mol Biol. 1986 Oct 5;191(3):411–420. doi: 10.1016/0022-2836(86)90136-1. [DOI] [PubMed] [Google Scholar]
- Holsters M., Silva B., Genetello C., Engler G., van Vliet F., de Block M., Villarroel R., van Montagu M., Schell J. Spontaneous formation of cointegrates of the oncogenic Ti-plasmid and the wide-host-range P-plasmid RP4. Plasmid. 1978 Sep;1(4):456–467. doi: 10.1016/0147-619x(78)90004-5. [DOI] [PubMed] [Google Scholar]
- Leyva A., Palacios J. M., Mozo T., Ruiz-Argüeso T. Cloning and characterization of hydrogen uptake genes from Rhizobium leguminosarum. J Bacteriol. 1987 Nov;169(11):4929–4934. doi: 10.1128/jb.169.11.4929-4934.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masterson R. V., Prakash R. K., Atherly A. G. Conservation of symbiotic nitrogen fixation gene sequences in Rhizobium japonicum and Bradyrhizobium japonicum. J Bacteriol. 1985 Jul;163(1):21–26. doi: 10.1128/jb.163.1.21-26.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Mulligan J. T., Long S. R. Induction of Rhizobium meliloti nodC expression by plant exudate requires nodD. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6609–6613. doi: 10.1073/pnas.82.19.6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankhurst C. E., Broughton W. J., Wieneke U. Transfer of an indigenous plasmid of Rhizobium loti to other rhizobia and Agrobacterium tumefaciens. J Gen Microbiol. 1983 Aug;129(8):2535–2543. doi: 10.1099/00221287-129-8-2535. [DOI] [PubMed] [Google Scholar]
- Prakash R. K., Atherly A. G. Reiteration of genes involved in symbiotic nitrogen fixation by fast-growing Rhizobium japonicum. J Bacteriol. 1984 Nov;160(2):785–787. doi: 10.1128/jb.160.2.785-787.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riess G., Holloway B. W., Pühler A. R68.45, a plasmid with chromosome mobilizing ability (Cma) carries a tandem duplication. Genet Res. 1980 Aug;36(1):99–109. doi: 10.1017/s0016672300019704. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg C., Boistard P., Dénarié J., Casse-Delbart F. Genes controlling early and late functions in symbiosis are located on a megaplasmid in Rhizobium meliloti. Mol Gen Genet. 1981;184(2):326–333. doi: 10.1007/BF00272926. [DOI] [PubMed] [Google Scholar]
- Rosenberg C., Casse-Delbart F., Dusha I., David M., Boucher C. Megaplasmids in the plant-associated bacteria Rhizobium meliloti and Pseudomonas solanacearum. J Bacteriol. 1982 Apr;150(1):402–406. doi: 10.1128/jb.150.1.402-406.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. Interspecies homology of nitrogenase genes. Proc Natl Acad Sci U S A. 1980 Jan;77(1):191–195. doi: 10.1073/pnas.77.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield P. R., Watson J. M. DNA sequence of Rhizobium trifolii nodulation genes reveals a reiterated and potentially regulatory sequence preceding nodABC and nodFE. Nucleic Acids Res. 1986 Apr 11;14(7):2891–2903. doi: 10.1093/nar/14.7.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearman C. A., Rossen L., Johnston A. W., Downie J. A. The Rhizobium leguminosarum nodulation gene nodF encodes a polypeptide similar to acyl-carrier protein and is regulated by nodD plus a factor in pea root exudate. EMBO J. 1986 Apr;5(4):647–652. doi: 10.1002/j.1460-2075.1986.tb04262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]