Abstract
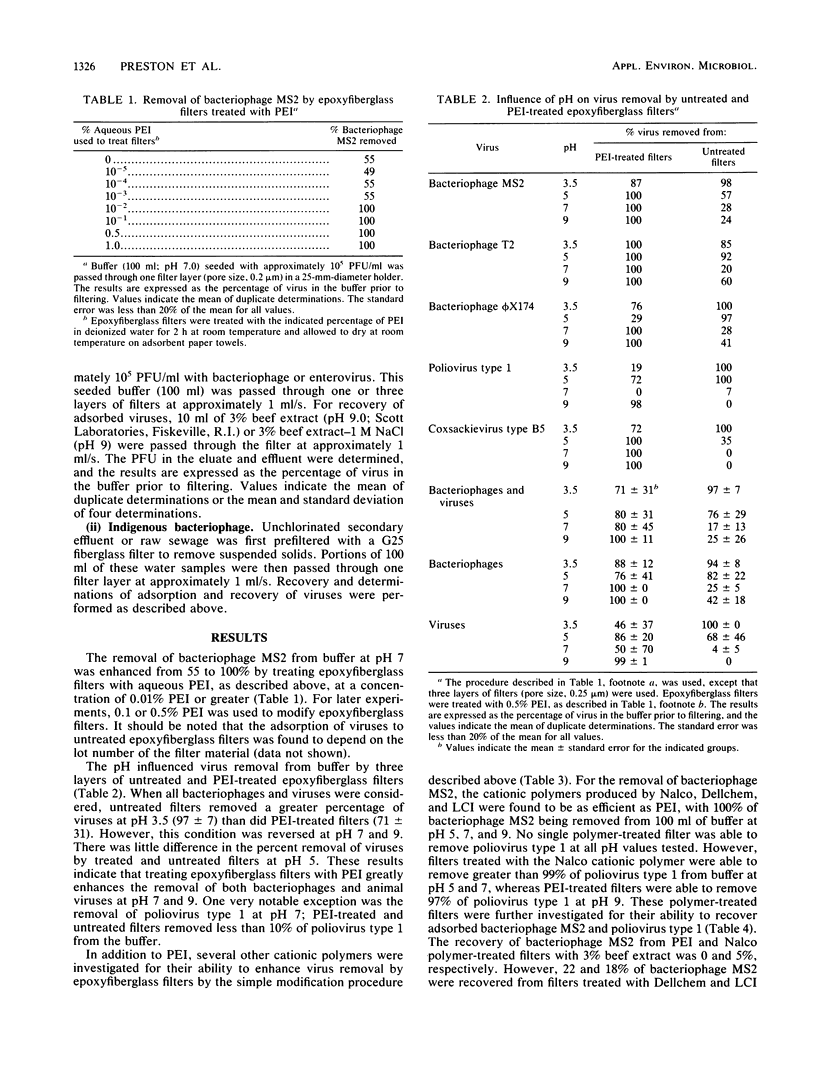
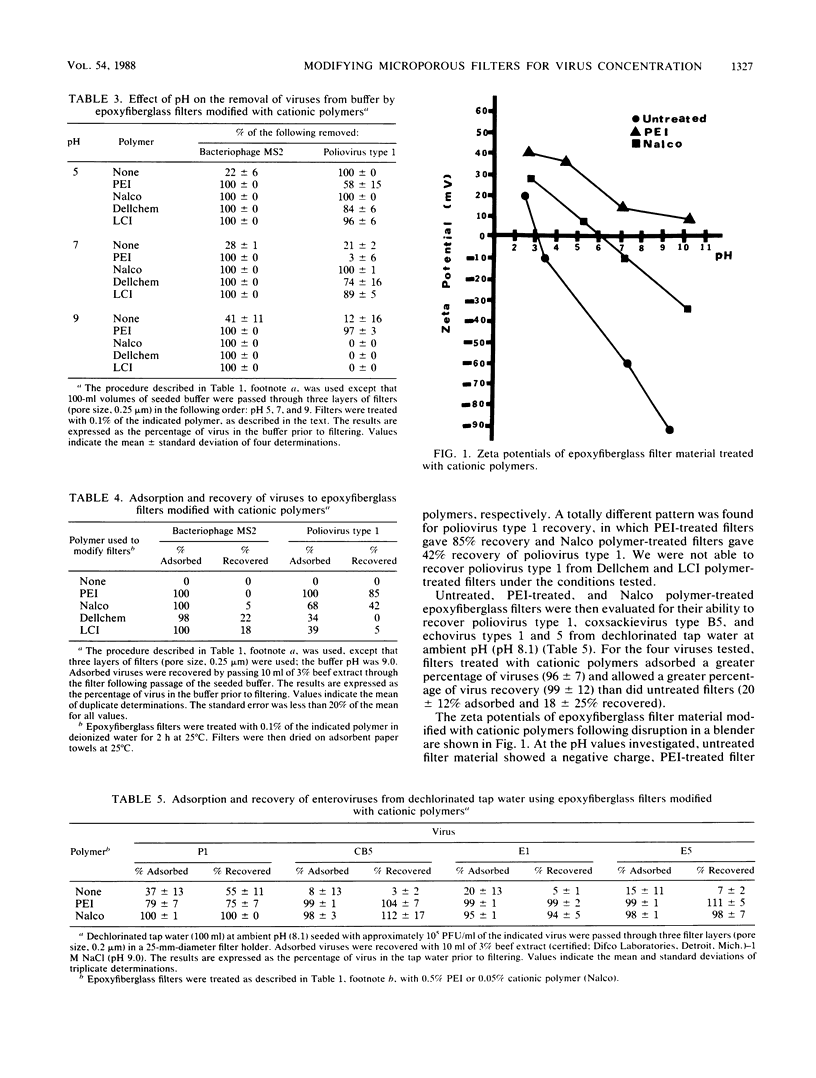
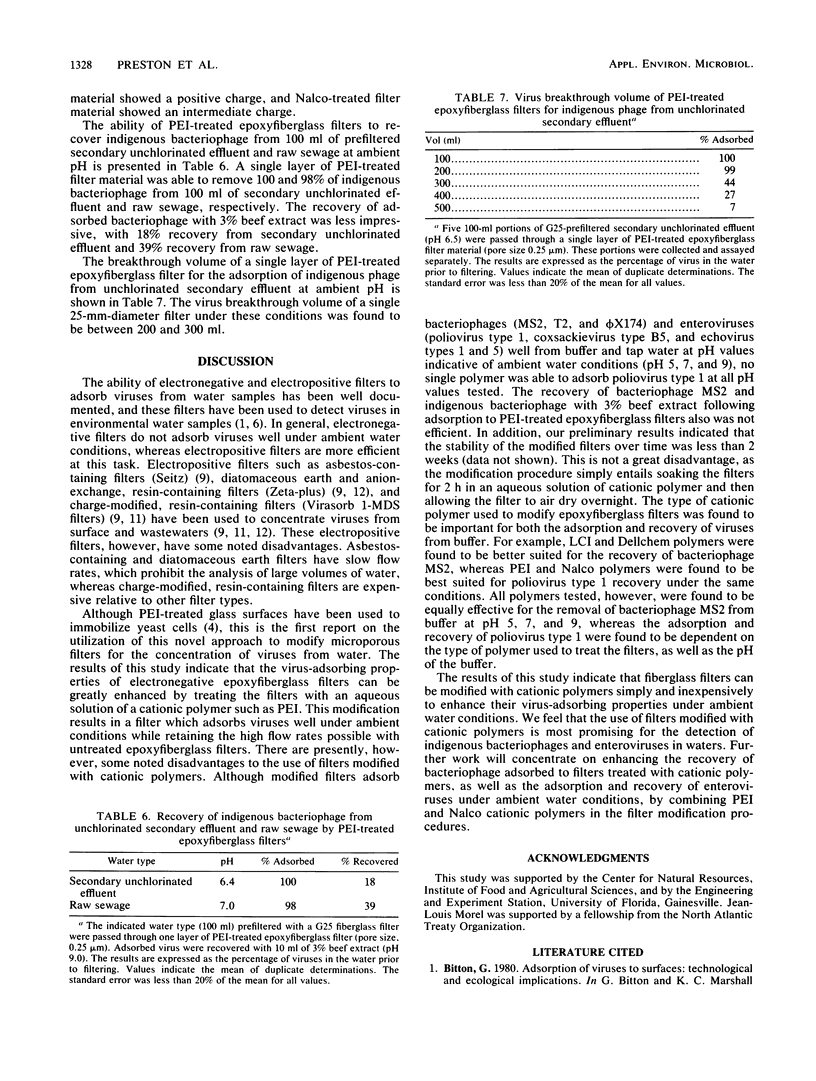
Electronegative microporous filters composed of epoxyfiberglass (Filterite) were treated with cationic polymers to enhance their virus-adsorbing properties. This novel and inexpensive approach to microporous filter modification entails soaking filters in an aqueous solution of a cationic polymer such as polyethyleneimine (PEI) for 2 h at room temperature and then allowing the filters to air dry overnight on absorbent paper towels. PEI-treated filters were evaluated for coliphage (MS2, T2, and phi X174) and enterovirus (poliovirus type 1 and coxsackievirus type B5) adsorption from buffer at pH 3.5 to 9.0 and for indigenous coliphages from unchlorinated secondary effluent at ambient pH. Adsorbed viruses were recovered with 3% beef extract (pH 9). Several other cationic polymers were used to modify epoxyfiberglass filters and were evaluated for their ability to concentrate viruses from water. Zeta potentials of disrupted filter material indicated that electronegative epoxyfiberglass filters were made more electropositive when treated with cationic polymers. In general, epoxyfiberglass filters treated with cationic polymers were found to adsorb a greater percentage of coliphages and enteroviruses than were untreated filters.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang L. T., Farrah S. R., Bitton G. Positively charged filters for virus recovery from wastewater treatment plant effluents. Appl Environ Microbiol. 1981 Nov;42(5):921–924. doi: 10.1128/aem.42.5.921-924.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerba C. P. Applied and theoretical aspects of virus adsorption to surfaces. Adv Appl Microbiol. 1984;30:133–168. doi: 10.1016/s0065-2164(08)70054-6. [DOI] [PubMed] [Google Scholar]
- Payment P., Trudel M., Sattar S. A., Springthorpe V. S., Subrahmanyan T. P., Gregory B. E., Vajdic A. H., Blaskovic P., Guglielmi I. J., Kudrewko O. Virological examination of drinking water: a Canadian collaborative study. Can J Microbiol. 1984 Jan;30(1):105–112. doi: 10.1139/m84-018. [DOI] [PubMed] [Google Scholar]
- Reid I. D. Biological Delignification of Aspen Wood by Solid-State Fermentation with the White-Rot Fungus Merulius tremellosus. Appl Environ Microbiol. 1985 Jul;50(1):133–139. doi: 10.1128/aem.50.1.133-139.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields P. A., Farrah S. R. Influence of salts on electrostatic interactions between poliovirus and membrane filters. Appl Environ Microbiol. 1983 Feb;45(2):526–531. doi: 10.1128/aem.45.2.526-531.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobsey M. D., Glass J. S. Poliovirus concentration from tap water with electropositive adsorbent filters. Appl Environ Microbiol. 1980 Aug;40(2):201–210. doi: 10.1128/aem.40.2.201-210.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobsey M. D., Jones B. L. Concentration of poliovirus from tap water using positively charged microporous filters. Appl Environ Microbiol. 1979 Mar;37(3):588–595. doi: 10.1128/aem.37.3.588-595.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerda K. S., Gerba C. P., Hou K. C., Goyal S. M. Adsorption of viruses to charge-modified silica. Appl Environ Microbiol. 1985 Jan;49(1):91–95. doi: 10.1128/aem.49.1.91-95.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


