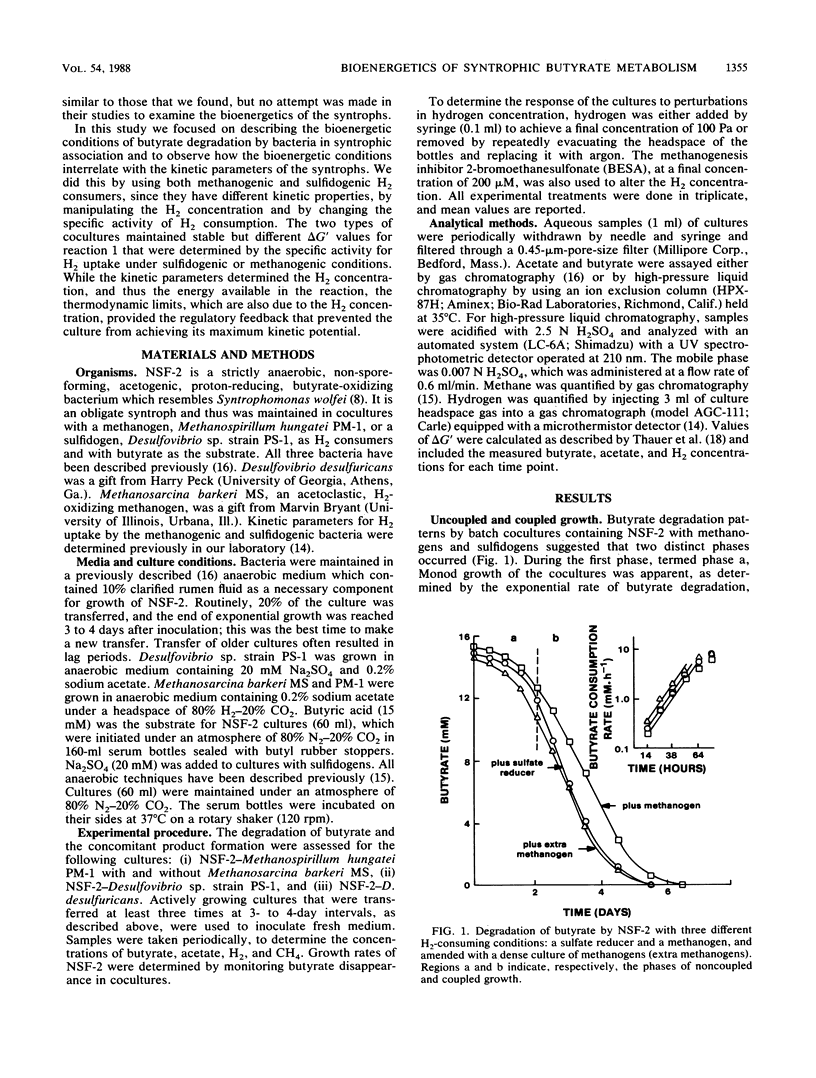
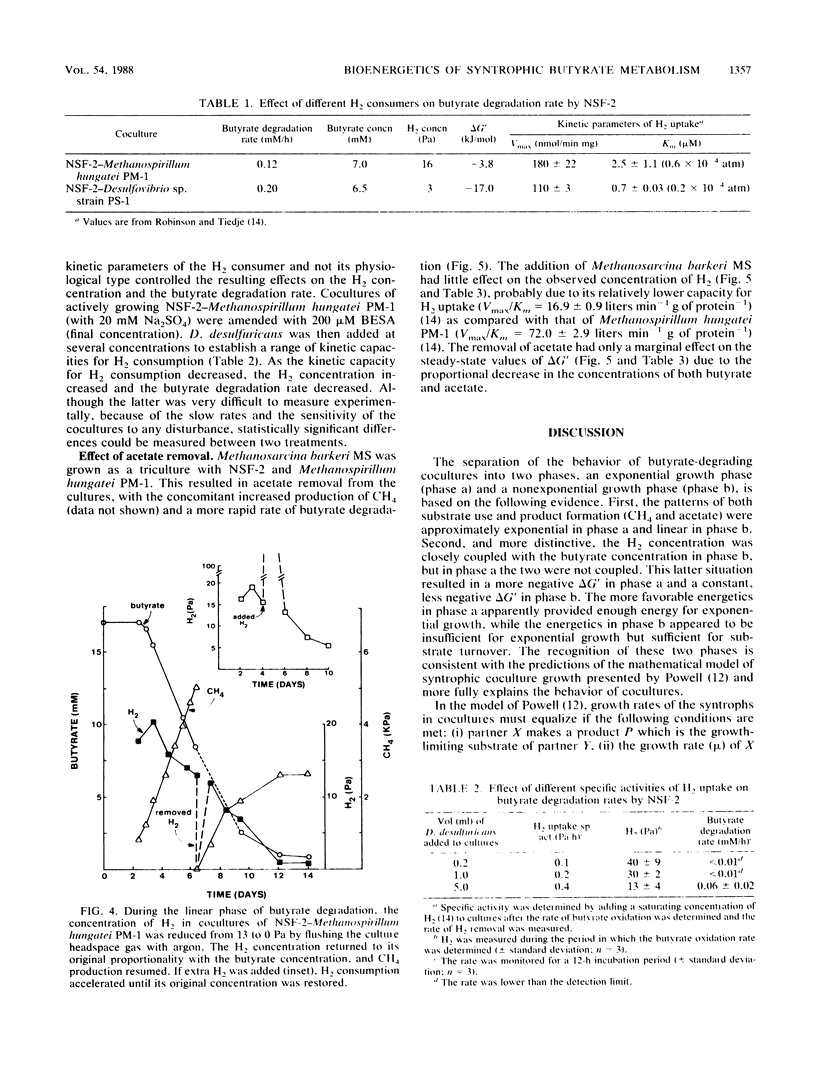
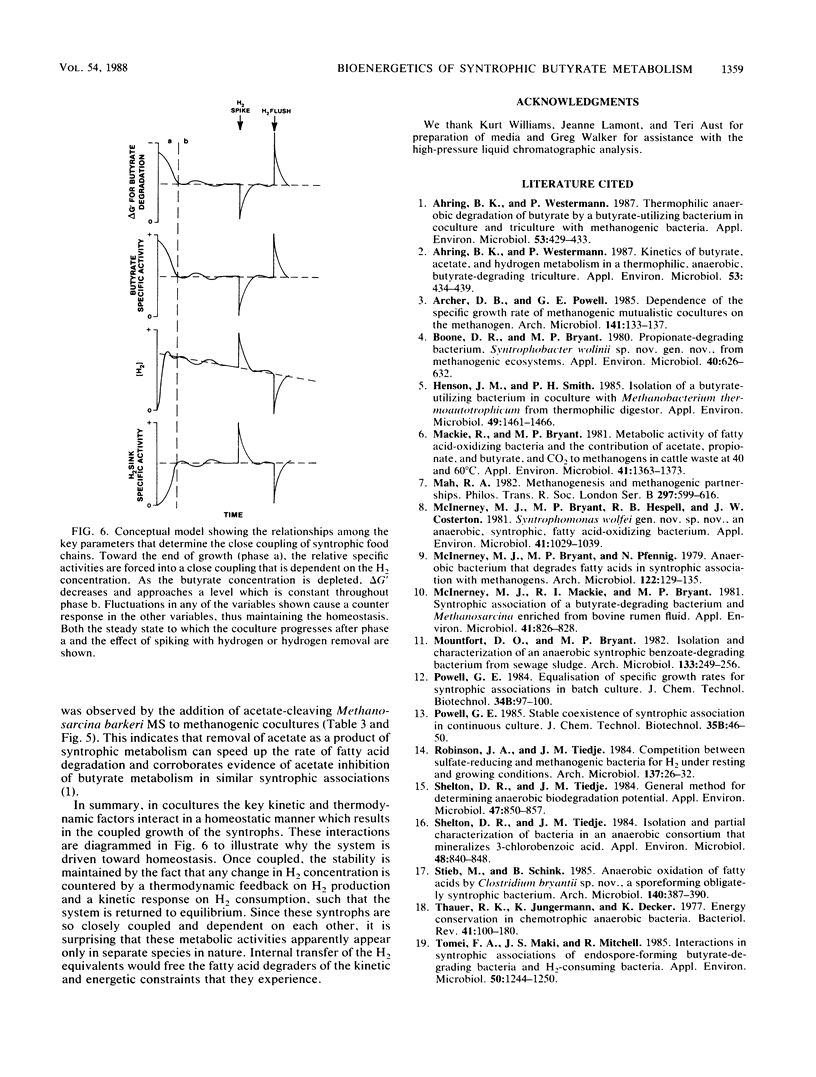
Abstract
The butyrate-oxidizing, proton-reducing, obligately anaerobic bacterium NSF-2 was grown in batch cocultures with either the hydrogen-oxidizing bacterium Methanospirillum hungatei PM-1 or Desulfovibrio sp. strain PS-1. Metabolism of butyrate occurred in two phases. The first phase exhibited exponential growth kinetics (phase a) and had a doubling time of 10 h. This value was independent of whether NSF-2 was cultured with a methanogen or a sulfate reducer and likely represents the maximum specific growth rate of NSF-2. This exponential growth phase was followed by a second phase with a nearly constant rate of degradation (phase b) which dominated the time course of butyrate degradation. The specific activity of H2 uptake by the hydrogen-oxidizing bacterium controlled the bioenergetic conditions of metabolism in phase b. During this phase both the Gibbs free energy (ΔG′) and the butyrate degradation rate (v) were greater for NSF-2-Desulfovibrio sp. strain PS-1 (ΔG′ = −17.0 kJ/mol; v = 0.20 mM/h) than for NSF-2-M. hungatei PM-1 (ΔG′ = −3.8 kJ/mol, v = 0.12 mM/h). The ΔG′ value remained stable and characteristic of the two hydrogen oxidizers during phase b. The stable ΔG′ resulted from the close coupling of the rates of butyrate and H2 oxidation. The addition of 2-bromoethanesulfonate to a NSF-2-methanogen coculture resulted in the total inhibition of butyrate degradation; the inhibition was relieved when Desulfovibrio sp. strain PS-1 was added as a new H2 sink. When the specific activity of H2 consumption was increased by adding higher densities of the Desulfovibrio sp. to 2-bromoethanesulfonate-inhibited NSF-2-methanogen cocultures, lower H2 pool sizes and higher rates of butyrate degradation resulted. Thus, it is the kinetic parameters of H2 consumption, not the type of H2 consumer per se, that establishes the thermodynamic conditions which in turn control the rate of fatty acid degradation. The bioenergetic homeostasis we observed in phase b was a result of the kinetics of the coculture members and the feedback inhibition by hydrogen which prevents butyrate degradation rates from reaching their theoretical Vmax.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahring B. K., Westermann P. Kinetics of butyrate, acetate, and hydrogen metabolism in a thermophilic, anaerobic, butyrate-degrading triculture. Appl Environ Microbiol. 1987 Feb;53(2):434–439. doi: 10.1128/aem.53.2.434-439.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahring B. K., Westermann P. Thermophilic anaerobic degradation of butyrate by a butyrate-utilizing bacterium in coculture and triculture with methanogenic bacteria. Appl Environ Microbiol. 1987 Feb;53(2):429–433. doi: 10.1128/aem.53.2.429-433.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone D. R., Bryant M. P. Propionate-Degrading Bacterium, Syntrophobacter wolinii sp. nov. gen. nov., from Methanogenic Ecosystems. Appl Environ Microbiol. 1980 Sep;40(3):626–632. doi: 10.1128/aem.40.3.626-632.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson J. M., Smith P. H. Isolation of a Butyrate-Utilizing Bacterium in Coculture with Methanobacterium thermoautotrophicum from a Thermophilic Digester. Appl Environ Microbiol. 1985 Jun;49(6):1461–1466. doi: 10.1128/aem.49.6.1461-1466.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie R. I., Bryant M. P. Metabolic Activity of Fatty Acid-Oxidizing Bacteria and the Contribution of Acetate, Propionate, Butyrate, and CO(2) to Methanogenesis in Cattle Waste at 40 and 60 degrees C. Appl Environ Microbiol. 1981 Jun;41(6):1363–1373. doi: 10.1128/aem.41.6.1363-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInerney M. J., Bryant M. P., Hespell R. B., Costerton J. W. Syntrophomonas wolfei gen. nov. sp. nov., an Anaerobic, Syntrophic, Fatty Acid-Oxidizing Bacterium. Appl Environ Microbiol. 1981 Apr;41(4):1029–1039. doi: 10.1128/aem.41.4.1029-1039.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInerney M. J., Mackie R. I., Bryant M. P. Syntrophic association of a butyrate-degrading bacterium and methanosarcina enriched from bovine rumen fluid. Appl Environ Microbiol. 1981 Mar;41(3):826–828. doi: 10.1128/aem.41.3.826-828.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton D. R., Tiedje J. M. General method for determining anaerobic biodegradation potential. Appl Environ Microbiol. 1984 Apr;47(4):850–857. doi: 10.1128/aem.47.4.850-857.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton D. R., Tiedje J. M. Isolation and partial characterization of bacteria in an anaerobic consortium that mineralizes 3-chlorobenzoic Acid. Appl Environ Microbiol. 1984 Oct;48(4):840–848. doi: 10.1128/aem.48.4.840-848.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomei F. A., Maki J. S., Mitchell R. Interactions in syntrophic associations of endospore-forming, butyrate-degrading bacteria and h(2)-consuming bacteria. Appl Environ Microbiol. 1985 Nov;50(5):1244–1250. doi: 10.1128/aem.50.5.1244-1250.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


