Abstract
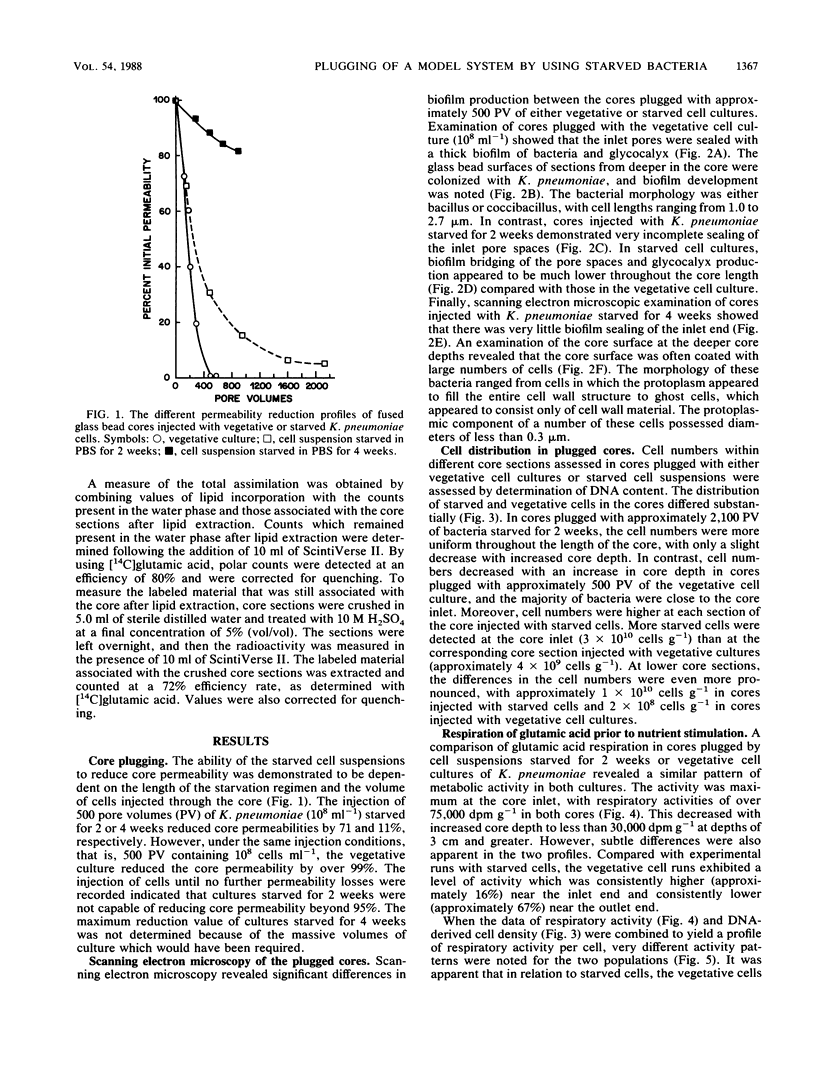
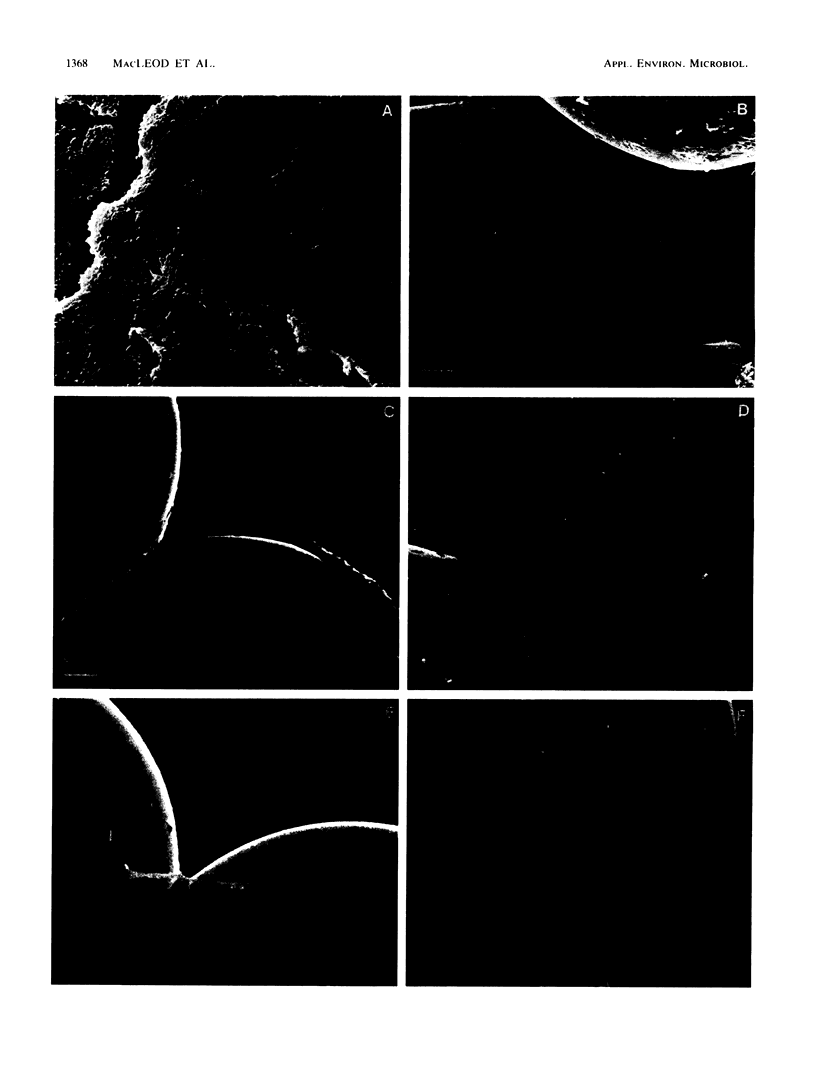
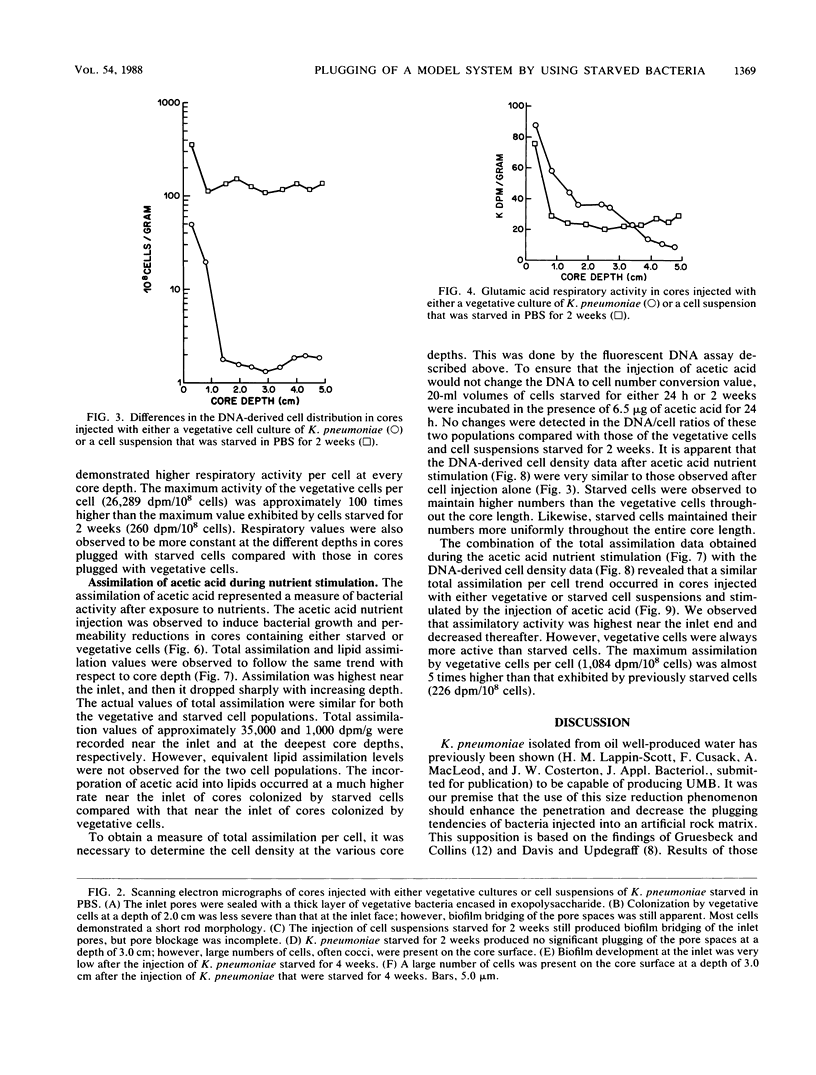
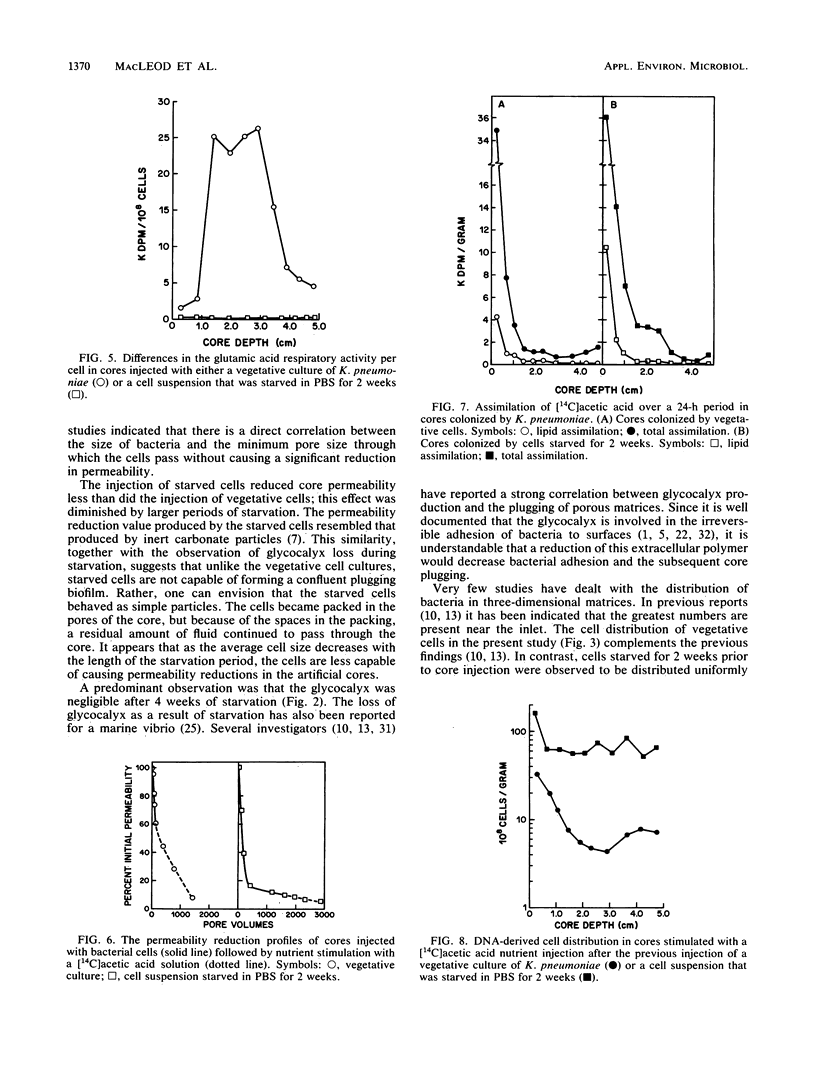
The effects of starvation on bacterial penetration through artificial rock cores were examined. Klebsiella pneumoniae was starved in a simple salts solution for a duration of up to 4 weeks. These cell suspensions were injected into sintered glass bead cores, and the resulting reductions in core permeabilities were recorded. Vegetative cell cultures of K. pneumoniae grown in a sodium citrate medium were injected into other, similar cores, and the reductions in core permeabilities were recorded. The starved cell suspensions did not completely block the core pores, whereas the vegetative cultures reduced core permeability to less than 1%. Scanning electron microscopy of core sections infiltrated with either vegetative or starved cells showed that the former produced shallow “skin” plugs and copious amounts of glycocalyx at the inlet face, whereas the latter produced very little glycocalyx and the cells were distributed evenly throughout the length of the core. The use of a DNA assay to produce a cell distribution profile showed that, compared with the vegetative cells, starved bacteria were able to penetrate deeper into the cores. This was due to the smaller size of the cells and the reduction in biofilm production. This ability of starved bacteria to penetrate further into cores than the normal-size vegetative cells can be usefully applied to selective plugging for enhanced oil recovery. To further test the suitability of starved cells for use in selective plugging, the activities of starved cells present within cores were monitored before and after nutrient stimulation. Our data indicate that with nutrient stimulation, the starved cells lose their metabolic dormancy and produce reductions in core permeability due to cell growth and polymer production.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bae H. C., Cota-Robles E. H., Casida L. E. Microflora of soil as viewed by transmission electron microscopy. Appl Microbiol. 1972 Mar;23(3):637–648. doi: 10.1128/am.23.3.637-648.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R. M., Singleton F. L., Hood M. A. Effects of nutrient deprivation on Vibrio cholerae. Appl Environ Microbiol. 1983 Oct;46(4):930–940. doi: 10.1128/aem.46.4.930-940.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Irvin R. T., Cheng K. J. The bacterial glycocalyx in nature and disease. Annu Rev Microbiol. 1981;35:299–324. doi: 10.1146/annurev.mi.35.100181.001503. [DOI] [PubMed] [Google Scholar]
- DAVIS J. B., UPDEGRAFF D. M. Microbiology in the petroleum industry. Bacteriol Rev. 1954 Dec;18(4):215–238. doi: 10.1128/br.18.4.215-238.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Novitsky J. A., Morita R. Y. Survival of a psychrophilic marine Vibrio under long-term nutrient starvation. Appl Environ Microbiol. 1977 Mar;33(3):635–641. doi: 10.1128/aem.33.3.635-641.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OPPENHEIMER C. H. The membrane filter in marine microbiology. J Bacteriol. 1952 Dec;64(6):783–786. doi: 10.1128/jb.64.6.783-786.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J. H., Myers B. Fluorometric determination of DNA in aquatic microorganisms by use of hoechst 33258. Appl Environ Microbiol. 1982 Jun;43(6):1393–1399. doi: 10.1128/aem.43.6.1393-1399.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. C., Bramhill B., Wardlaw N. C., Costerton J. W. Bacterial fouling in a model core system. Appl Environ Microbiol. 1985 Mar;49(3):693–701. doi: 10.1128/aem.49.3.693-701.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrella F., Morita R. Y. Microcultural study of bacterial size changes and microcolony and ultramicrocolony formation by heterotrophic bacteria in seawater. Appl Environ Microbiol. 1981 Feb;41(2):518–527. doi: 10.1128/aem.41.2.518-527.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyndham R. C., Costerton J. W. Heterotrophic potentials and hydrocarbon biodegradation potentials of sediment microorganisms within the athabasca oil sands deposit. Appl Environ Microbiol. 1981 Mar;41(3):783–790. doi: 10.1128/aem.41.3.783-790.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]




