Abstract
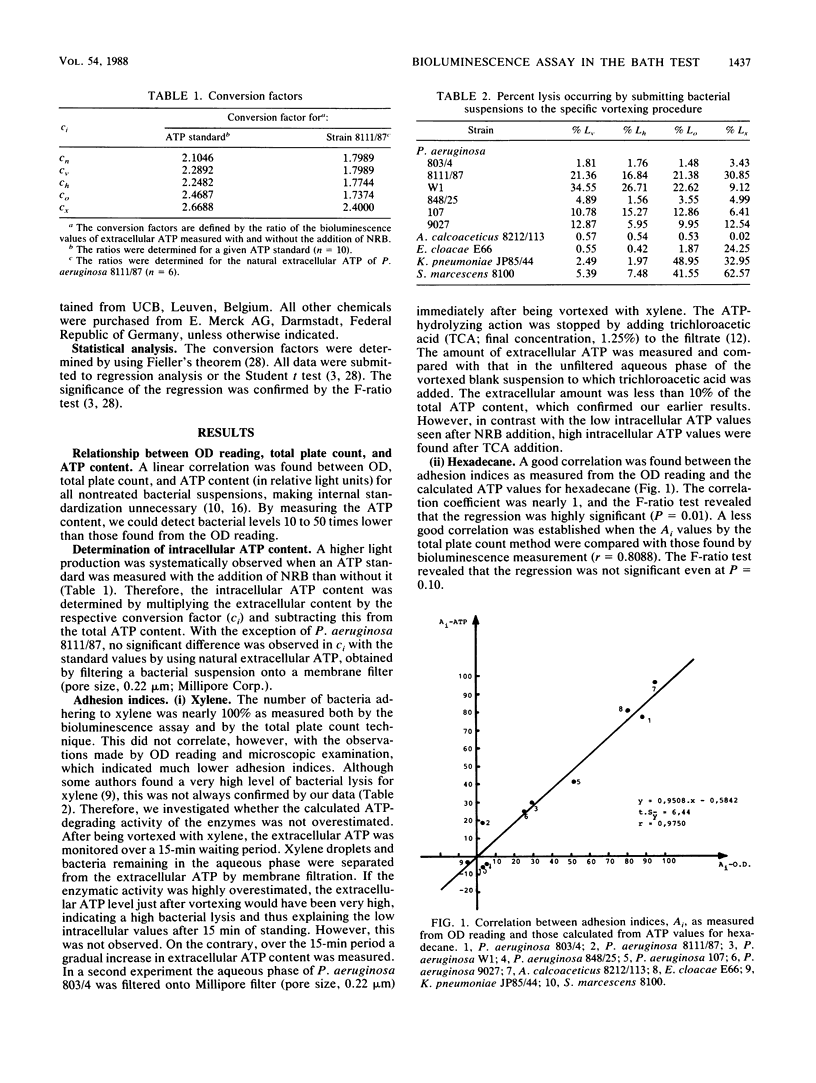
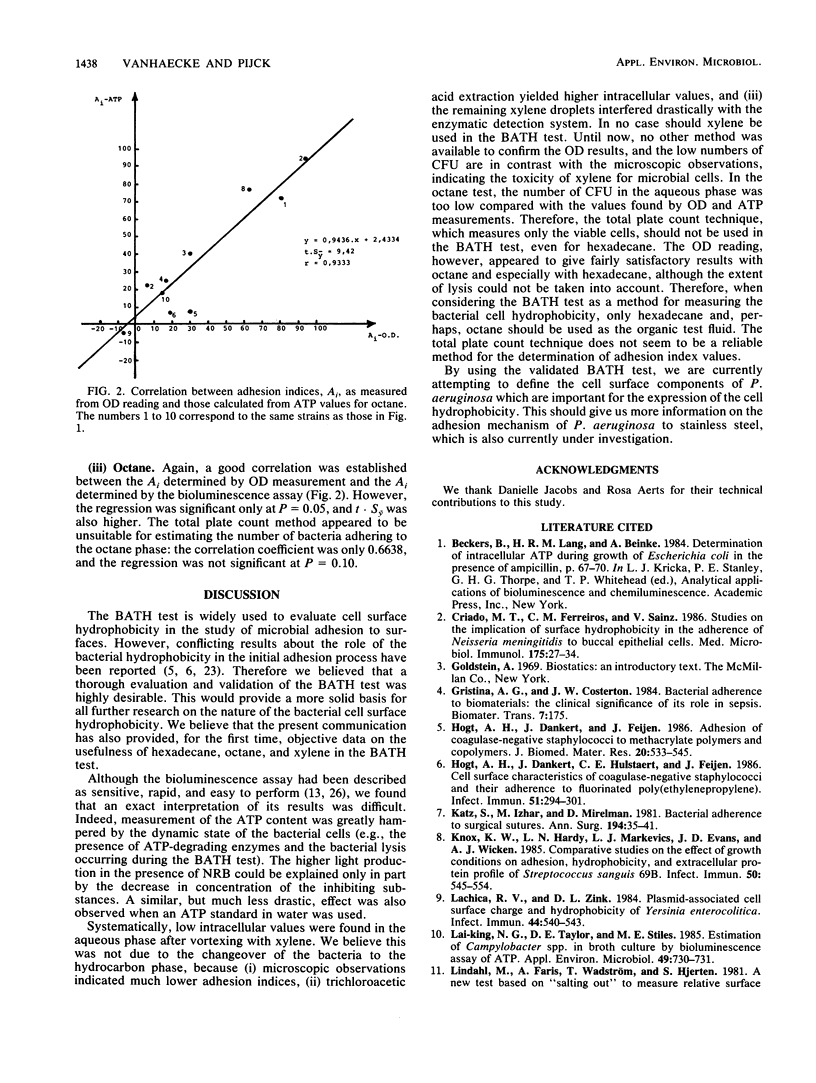
A thorough validation of the bacterial adherence to hydrocarbons (BATH) test was performed by means of a bioluminescence assay. Ten different gram-negative strains were subjected to the BATH test. For the calculation of the adhesion index, several factors had to be taken into account: ATP leakage, the action of ATP-hydrolyzing enzymes, the change in the extraction efficiency of Nucleotide-Releasing Reagent for Microbial Cells (NRB; Lumac bv) after vortexing and the difference in light production after the addition of NRB. When the adhesion index values obtained by bioluminescence measurement were used as reference, the total plate count technique appeared to be unreliable in estimating the number of bacteria adhering to the hydrocarbon phase. A highly significant correlation was established, however, between those reference values and the adhesion index values obtained by the optical density reading for octane and especially for hexadecane. With xylene, no correlation was found between the optical density reading values and the total plate count or bioluminescence values.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Criado M. T., Ferreiros C. M., Sainz V. Studies on the implication of surface hydrophobicity in the adherence of Neisseria meningitidis to buccal epithelial cells. Med Microbiol Immunol. 1986;175(1):27–34. doi: 10.1007/BF02123126. [DOI] [PubMed] [Google Scholar]
- Hogt A. H., Dankert J., Feijen J. Adhesion of coagulase-negative staphylococci to methacrylate polymers and copolymers. J Biomed Mater Res. 1986 Apr;20(4):533–545. doi: 10.1002/jbm.820200409. [DOI] [PubMed] [Google Scholar]
- Hogt A. H., Dankert J., Hulstaert C. E., Feijen J. Cell surface characteristics of coagulase-negative staphylococci and their adherence to fluorinated poly(ethylenepropylene). Infect Immun. 1986 Jan;51(1):294–301. doi: 10.1128/iai.51.1.294-301.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz S., Izhar M., Mirelman D. Bacterial adherence to surgical sutures. A possible factor in suture induced infection. Ann Surg. 1981 Jul;194(1):35–41. doi: 10.1097/00000658-198107000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Hardy L. N., Markevics L. J., Evans J. D., Wicken A. J. Comparative studies on the effect of growth conditions on adhesion, hydrophobicity, and extracellular protein profile of Streptococcus sanguis G9B. Infect Immun. 1985 Nov;50(2):545–554. doi: 10.1128/iai.50.2.545-554.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachica R. V., Zink D. L. Plasmid-associated cell surface charge and hydrophobicity of Yersinia enterocolitica. Infect Immun. 1984 May;44(2):540–543. doi: 10.1128/iai.44.2.540-543.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl M., Faris A., Wadström T., Hjertén S. A new test based on 'salting out' to measure relative surface hydrophobicity of bacterial cells. Biochim Biophys Acta. 1981 Nov 5;677(3-4):471–476. doi: 10.1016/0304-4165(81)90261-0. [DOI] [PubMed] [Google Scholar]
- McBride B. C., Song M., Krasse B., Olsson J. Biochemical and immunological differences between hydrophobic and hydrophilic strains of Streptococcus mutans. Infect Immun. 1984 Apr;44(1):68–75. doi: 10.1128/iai.44.1.68-75.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWalter P. W. Determination of susceptibility of Staphylococcus aureus to methicillin by luciferin-luciferase assay of bacterial adenosine triphosphate. J Appl Bacteriol. 1984 Feb;56(1):145–150. doi: 10.1111/j.1365-2672.1984.tb04706.x. [DOI] [PubMed] [Google Scholar]
- Ng L. K., Taylor D. E., Stiles M. E. Estimation of Campylobacter spp. in broth culture by bioluminescence assay of ATP. Appl Environ Microbiol. 1985 Mar;49(3):730–731. doi: 10.1128/aem.49.3.730-731.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchido T., Katsui N., Takeuchi A., Takano M., Shibasaki I. Destruction of the outer membrane permeability barrier of Escherichia coli by heat treatment. Appl Environ Microbiol. 1985 Aug;50(2):298–303. doi: 10.1128/aem.50.2.298-303.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oss C. J., Gillman C. F. Phagocytosis as a surface phenomenon. Contact angles and phagocytosis of non-opsonized bacteria. J Reticuloendothel Soc. 1972 Sep;12(3):283–292. [PubMed] [Google Scholar]
- Webster J. J., Chang J. C., Manley E. R., Spivey H. O., Leach F. R. Buffer effects on ATP analysis by firefly luciferase. Anal Biochem. 1980 Jul 15;106(1):7–11. doi: 10.1016/0003-2697(80)90111-6. [DOI] [PubMed] [Google Scholar]
- Wheeler T. T., Clark W. B., Lane M. D., Grow T. E. Influence of physicochemical parameters on adsorption of Actinomyces viscosus to hydroxyapatite surfaces. Infect Immun. 1983 Mar;39(3):1095–1101. doi: 10.1128/iai.39.3.1095-1101.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loosdrecht M. C., Lyklema J., Norde W., Schraa G., Zehnder A. J. The role of bacterial cell wall hydrophobicity in adhesion. Appl Environ Microbiol. 1987 Aug;53(8):1893–1897. doi: 10.1128/aem.53.8.1893-1897.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oss C. J. Phagocytosis as a surface phenomenon. Annu Rev Microbiol. 1978;32:19–39. doi: 10.1146/annurev.mi.32.100178.000315. [DOI] [PubMed] [Google Scholar]


