Abstract
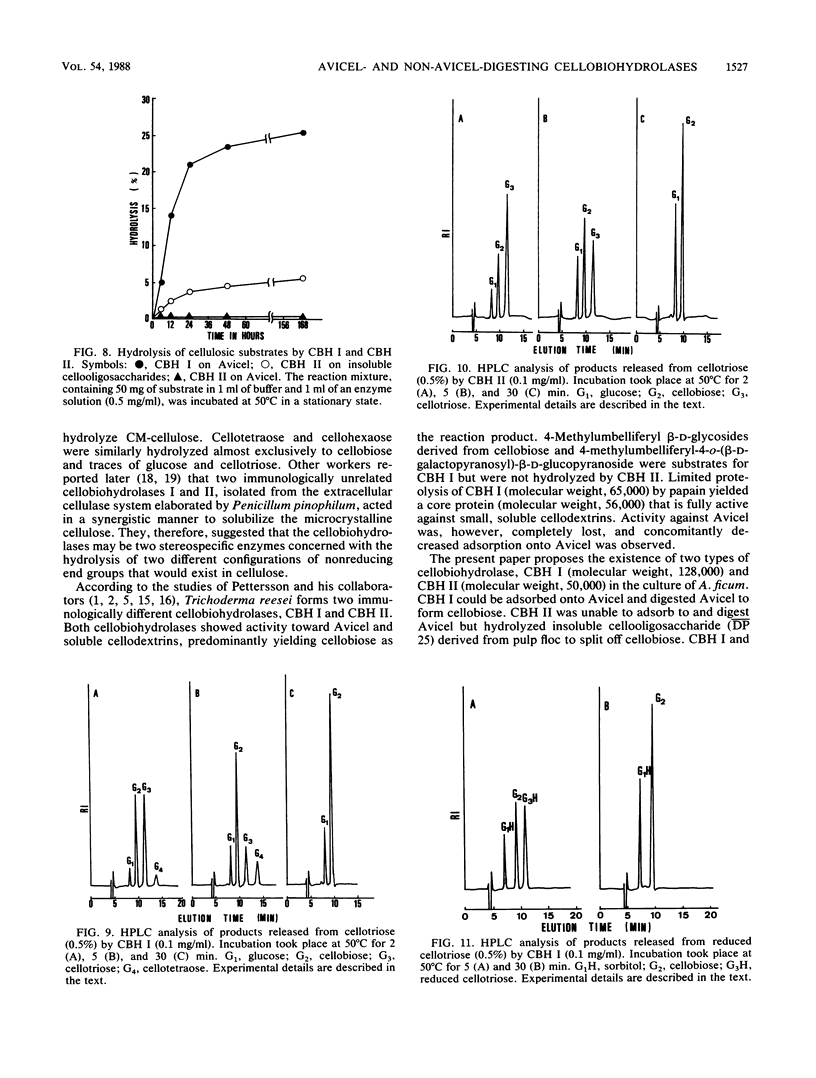
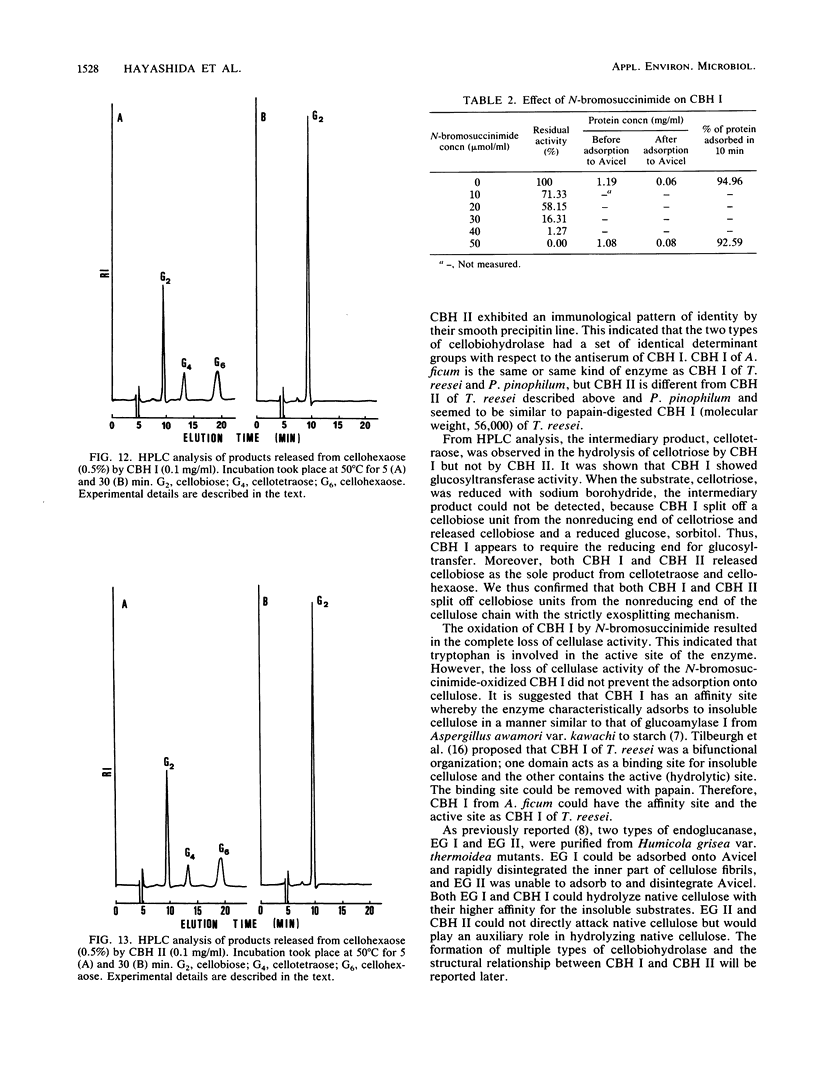
Two immunologically related cellobiohydrolases, cellobiohydrolase I (CBH I) and cellobiohydrolase II (CBH II), were purified from Aspergillus ficum. The Avicel-adsorbable CBH I (molecular weight, 128,000) digested Avicel, cotton, and cellulose powder to cellobiose, but the Avicel-unadsorbable CBH II (molecular weight, 50,000) could not digest those substrates. Both enzymes hydrolyzed insoluble cellooligosaccharides (D̄P̄ 25) to cellobiose. High-pressure liquid chromatographic analysis of soluble cellooligosaccharide hydrolysates revealed that both enzymes split off strictly cellobiose units from the nonreducing end of the cellulose chain with an exowise mechanism. CBH I showed glucosyltransferase activity, but CBH II did not. The N-bromosuccinimideoxidized CBH I was completely inactive but retained the ability to adsorb to Avicel. This suggested that CBH I has separate sites for binding to cellulose and for catalyzing cleavage of glycosidic linkages. Cellobiohydrolases were of two types, CBH I and CBH II. The former can adsorb to and digest Avicel, while the latter can do neither.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berghem L. E., Pettersson L. G. The mechanism of enzymatic cellulose degradation. Purification of a cellulolytic enzyme from Trichoderma viride active on highly ordered cellulose. Eur J Biochem. 1973 Aug 1;37(1):21–30. doi: 10.1111/j.1432-1033.1973.tb02952.x. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Halliwell G., Griffin M. The nature and mode of action of the cellulolytic component C1 of Trichoderma koningii on native cellulose. Biochem J. 1973 Dec;135(4):587–594. doi: 10.1042/bj1350587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida S., Mo K. Production and Characteristics of Avicel-Disintegrating Endoglucanase from a Protease-Negative Humicola grisea var. thermoidea Mutant. Appl Environ Microbiol. 1986 May;51(5):1041–1046. doi: 10.1128/aem.51.5.1041-1046.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wood T. M., McCrae S. I., Macfarlane C. C. The isolation, purification and properties of the cellobiohydrolase component of Penicillium funiculosum cellulase. Biochem J. 1980 Jul 1;189(1):51–65. doi: 10.1042/bj1890051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood T. M., McCrae S. I. The cellulase of Penicillium pinophilum. Synergism between enzyme components in solubilizing cellulose with special reference to the involvement of two immunologically distinct cellobiohydrolases. Biochem J. 1986 Feb 15;234(1):93–99. doi: 10.1042/bj2340093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood T. M., McCrae S. I. The purification and properties of the C 1 component of Trichoderma koningii cellulase. Biochem J. 1972 Aug;128(5):1183–1192. doi: 10.1042/bj1281183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tilbeurgh H., Pettersson G., Bhikabhai R., De Boeck H., Claeyssens M. Studies of the cellulolytic system of Trichoderma reesei QM 9414. Reaction specificity and thermodynamics of interactions of small substrates and ligands with the 1,4-beta-glucan cellobiohydrolase II. Eur J Biochem. 1985 Apr 15;148(2):329–334. doi: 10.1111/j.1432-1033.1985.tb08843.x. [DOI] [PubMed] [Google Scholar]