Abstract
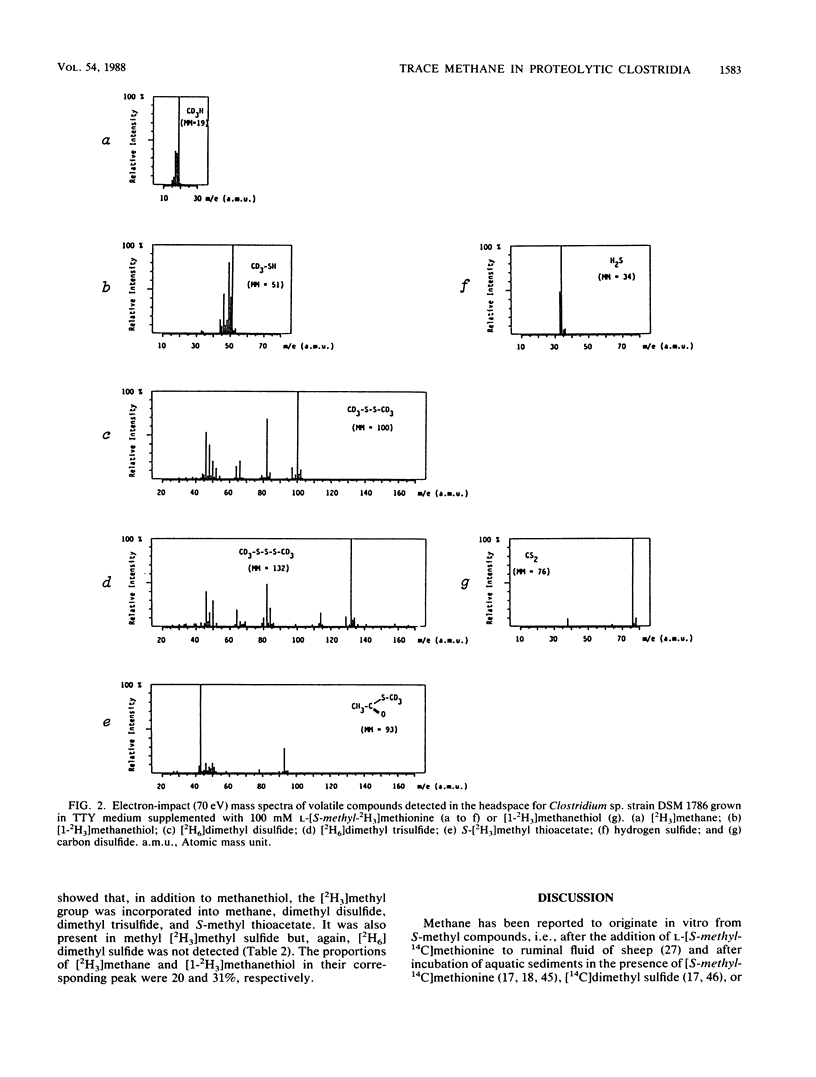
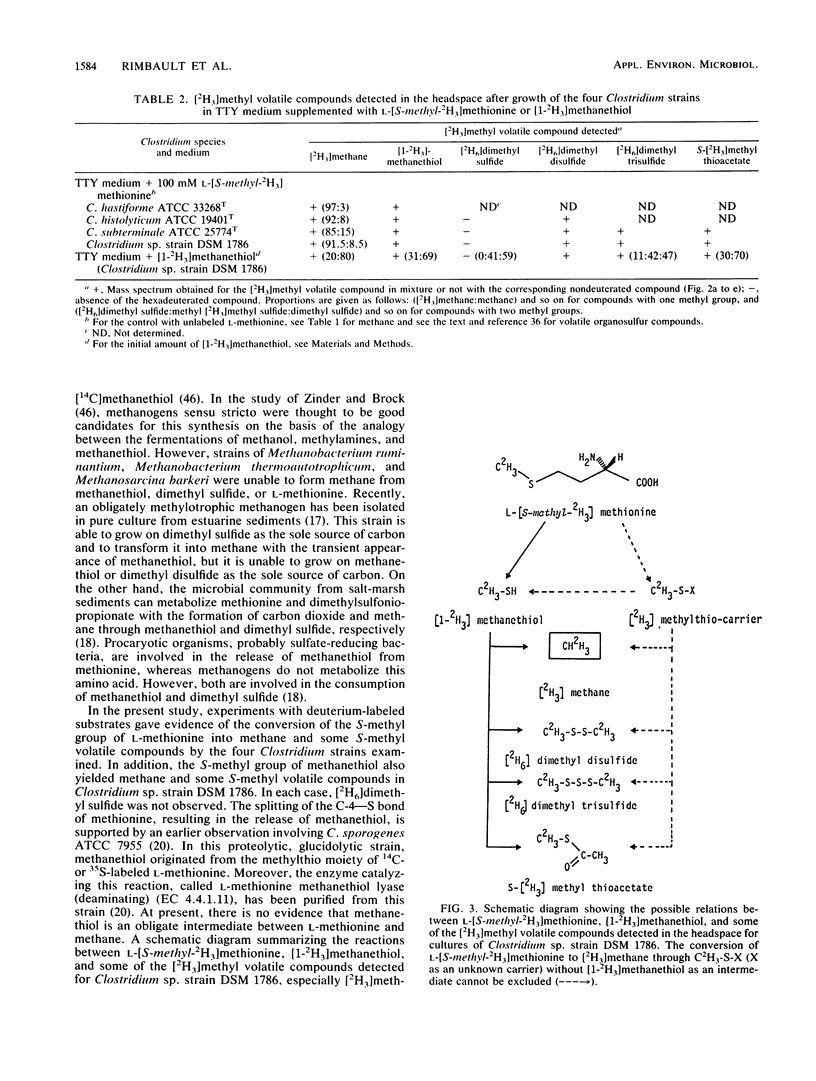
The in vivo formation of methane and of several S-methyl volatile compounds from the terminal S-methyl group of l-methionine is reported for growing cultures of four Clostridium strains (C. hastiforme, C. histolyticum, C. subterminale, and Clostridium sp. strain DSM 1786). After growth in 5 ml of unamended medium, C. hastiforme formed the highest amount of methane (408 nmol per tube in the headspace). When the culture medium was amended with 100 mM l-[S-methyl-2H3]methionine, the four strains formed [2H3]methane (proportion in the methane peak, >85%) as well as methanethiol, dimethyl disulfide, dimethyl trisulfide, and S-methyl thioacetate labeled on the methyl moiety. Methanethiol is also a precursor of methane for Clostridium sp. strain DSM 1786. The trace methane formation observed for these four proteolytic, nonglucidolytic Clostridium strains can be of ecological interest, particularly in aquatic sediments and in the gastrointestinal tract of humans and animals. It can explain in part the trace methane formation which cannot be ascribed to methanogens sensu stricto.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cato E. P., Hash D. E., Holdeman L. V., Moore W. E. Electrophoretic study of Clostridium species. J Clin Microbiol. 1982 Apr;15(4):688–702. doi: 10.1128/jcm.15.4.688-702.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmiston C. E., Jr, Avant G. R., Wilson F. A. Anaerobic bacterial populations on normal and diseased human biopsy tissue obtained at colonoscopy. Appl Environ Microbiol. 1982 May;43(5):1173–1181. doi: 10.1128/aem.43.5.1173-1181.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines A., Metz G., Dilawari J., Blendis L., Wiggins H. Breath-methane in patients with cancer of the large bowel. Lancet. 1977 Sep 3;2(8036):481–483. doi: 10.1016/s0140-6736(77)91605-1. [DOI] [PubMed] [Google Scholar]
- Kadota H., Ishida Y. Production of volatile sulfur compounds by microorganisms. Annu Rev Microbiol. 1972;26:127–138. doi: 10.1146/annurev.mi.26.100172.001015. [DOI] [PubMed] [Google Scholar]
- Karlin D. A., Mastromarino A. J., Jones R. D., Stroehlein J. R., Lorentz O. Fecal skatole and indole and breath methane and hydrogen in patients with large bowel polyps or cancer. J Cancer Res Clin Oncol. 1985;109(2):135–141. doi: 10.1007/BF00391888. [DOI] [PubMed] [Google Scholar]
- Kiene R. P., Oremland R. S., Catena A., Miller L. G., Capone D. G. Metabolism of reduced methylated sulfur compounds in anaerobic sediments and by a pure culture of an estuarine methanogen. Appl Environ Microbiol. 1986 Nov;52(5):1037–1045. doi: 10.1128/aem.52.5.1037-1045.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiene R. P., Visscher P. T. Production and fate of methylated sulfur compounds from methionine and dimethylsulfoniopropionate in anoxic salt marsh sediments. Appl Environ Microbiol. 1987 Oct;53(10):2426–2434. doi: 10.1128/aem.53.10.2426-2434.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulkes-Pujo A. M., Moreau M., Sutton J. Methane formation from the reactions of hydroxyl radicals and hydrogen atoms with dimethyl sulfoxide (DMSO). FEBS Lett. 1981 Jun 29;129(1):52–54. doi: 10.1016/0014-5793(81)80753-3. [DOI] [PubMed] [Google Scholar]
- Kreis W., Hession C. Isolation and purification of L-methionine-alpha-deamino-gamma-mercaptomethane-lyase (L-methioninase) from Clostridium sporogenes. Cancer Res. 1973 Aug;33(8):1862–1865. [PubMed] [Google Scholar]
- Matches J. R., Liston J. Mesophilic clostridia in Puget Sound. Can J Microbiol. 1974 Jan;20(1):1–7. doi: 10.1139/m74-001. [DOI] [PubMed] [Google Scholar]
- McKay L. F., Holbrook W. P., Eastwood M. A. Methane and hydrogen production by human intestinal anaerobic bacteria. Acta Pathol Microbiol Immunol Scand B. 1982 Jun;90(3):257–260. doi: 10.1111/j.1699-0463.1982.tb00114.x. [DOI] [PubMed] [Google Scholar]
- Molongoski J. J., Klug M. J. Characterization of anaerobic heterotrophic bacteria isolated from freshwater lake sediments. Appl Environ Microbiol. 1976 Jan;31(1):83–90. doi: 10.1128/aem.31.1.83-90.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill A. R., Grime D. W., Dawson R. M. Conversion of choline methyl groups through trimethylamine into methane in the rumen. Biochem J. 1978 Mar 15;170(3):529–535. doi: 10.1042/bj1700529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S., Zehr J. P. Formation of methane and carbon dioxide from dimethylselenide in anoxic sediments and by a methanogenic bacterium. Appl Environ Microbiol. 1986 Nov;52(5):1031–1036. doi: 10.1128/aem.52.5.1031-1036.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons J. L., Rimbault A., Darbord J. C., Leluan G. Biosynthèse de toluène chez Clostridium aerofoetidum souche WS. Ann Microbiol (Paris) 1984 Sep-Oct;135B(2):219–222. [PubMed] [Google Scholar]
- Pons J. L., Rimbault A., Darbord J. C., Leluan G. Gas chromatographic-mass spectrometric analysis of volatile amines produced by several strains of Clostridium. J Chromatogr. 1985 Feb 8;337(2):213–221. doi: 10.1016/0378-4347(85)80034-7. [DOI] [PubMed] [Google Scholar]
- Postgate J. R. Methane as a minor product of pyruvate metabolism by sulphate-reducing and other bacteria. J Gen Microbiol. 1969 Aug;57(3):293–302. doi: 10.1099/00221287-57-3-293. [DOI] [PubMed] [Google Scholar]
- Rimbault A., Leluan G. Composés neutres et basiques présents dans les gaz produits par Clostridium histolyticum, Clostridium hastiforme et Clostridium ghoni cultivés sous vide en milieu glucosé au thioglycolate de sodium. C R Seances Acad Sci III. 1982 Sep 27;295(3):219–221. [PubMed] [Google Scholar]
- Rimbault A., Leluan G. Composés neutres et basiques présents dans les gaz produits par Clostridium sporogenes, Plectridium putrificum et Plectridium glycolicum cultivés sous vide en milieu glucosé au thioglycolate de sodium. C R Seances Acad Sci III. 1982 Oct 4;295(4):299–302. [PubMed] [Google Scholar]
- Rimbault A., Niel P., Darbord J. C., Leluan G. Headspace gas chromatographic-mass spectrometric analysis of light hydrocarbons and volatile organosulphur compounds in reduced-pressure cultures of Clostridium. J Chromatogr. 1986 Feb 14;375(1):11–25. doi: 10.1016/s0378-4347(00)83687-7. [DOI] [PubMed] [Google Scholar]
- SISLER F. D., ZOBELL C. E. Hydrogen utilization by some marine sulfate-reducing bacteria. J Bacteriol. 1951 Jul;62(1):117–127. doi: 10.1128/jb.62.1.117-127.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G. Metabolism of one-carbon compounds by chemotrophic anaerobes. Adv Microb Physiol. 1983;24:215–299. doi: 10.1016/s0065-2911(08)60387-2. [DOI] [PubMed] [Google Scholar]
- Zinder S. H., Brock T. D. Methane, carbon dioxide, and hydrogen sulfide production from the terminal methiol group of methionine by anaerobic lake sediments. Appl Environ Microbiol. 1978 Feb;35(2):344–352. doi: 10.1128/aem.35.2.344-352.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]