Abstract
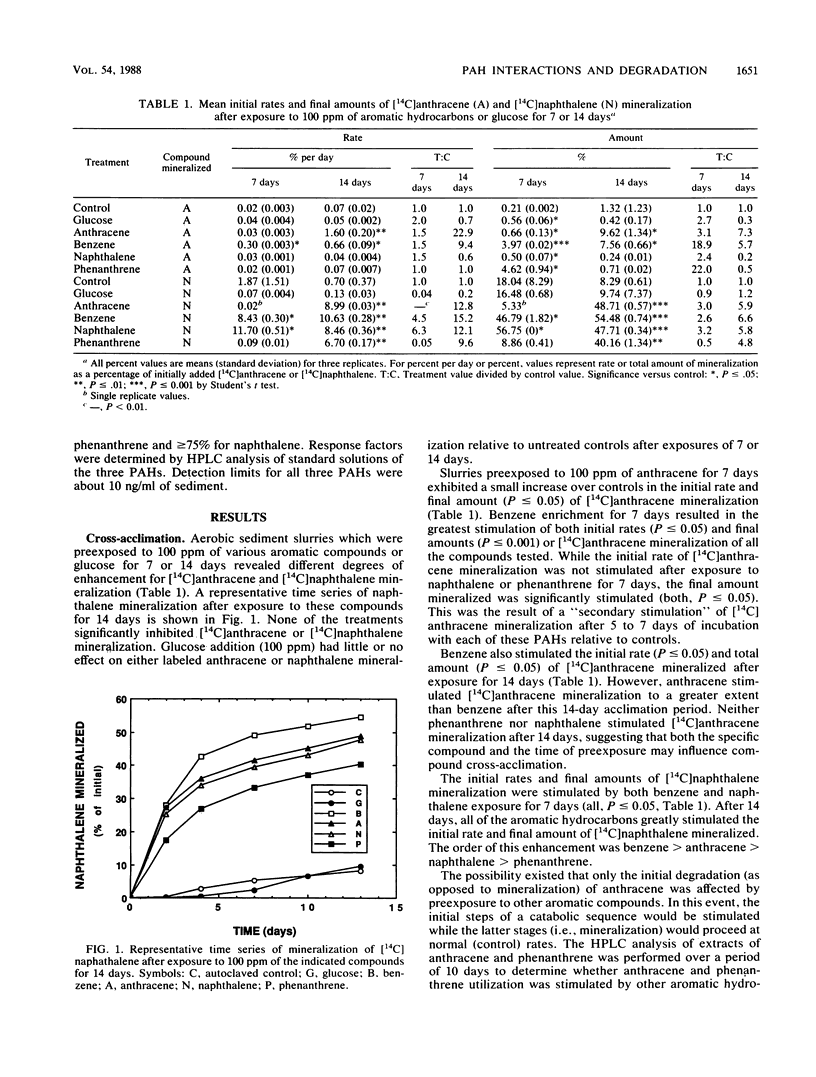
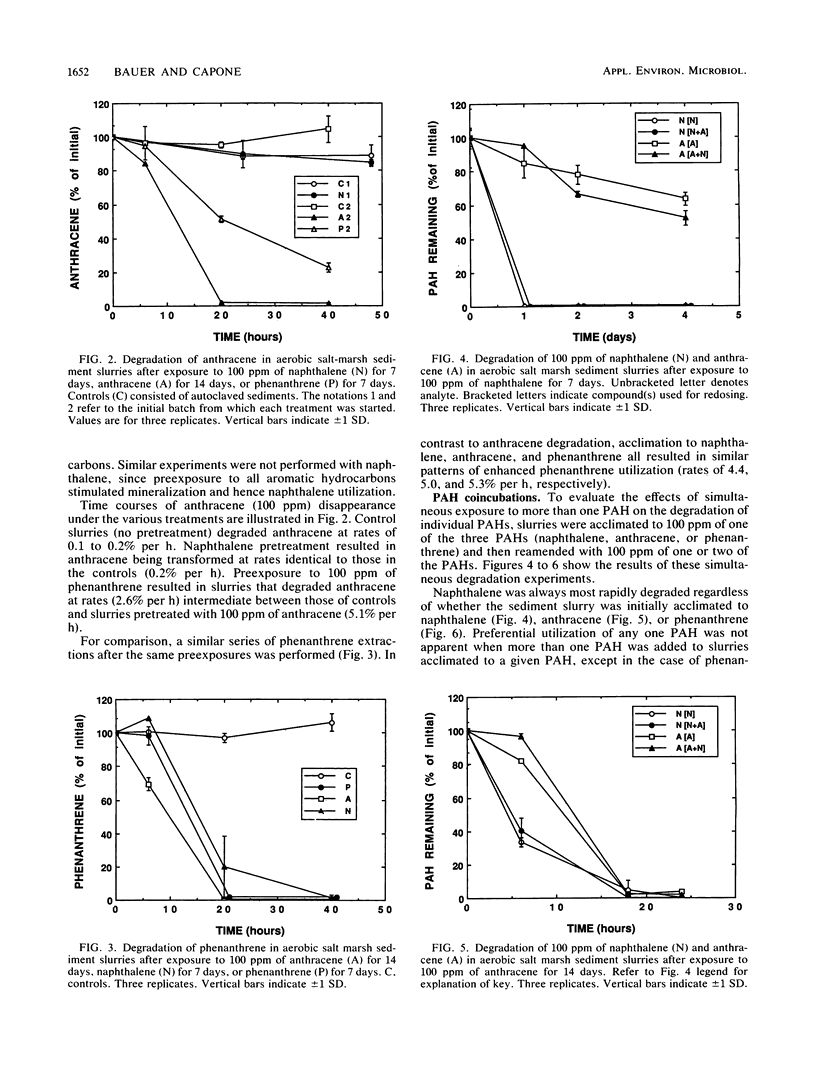
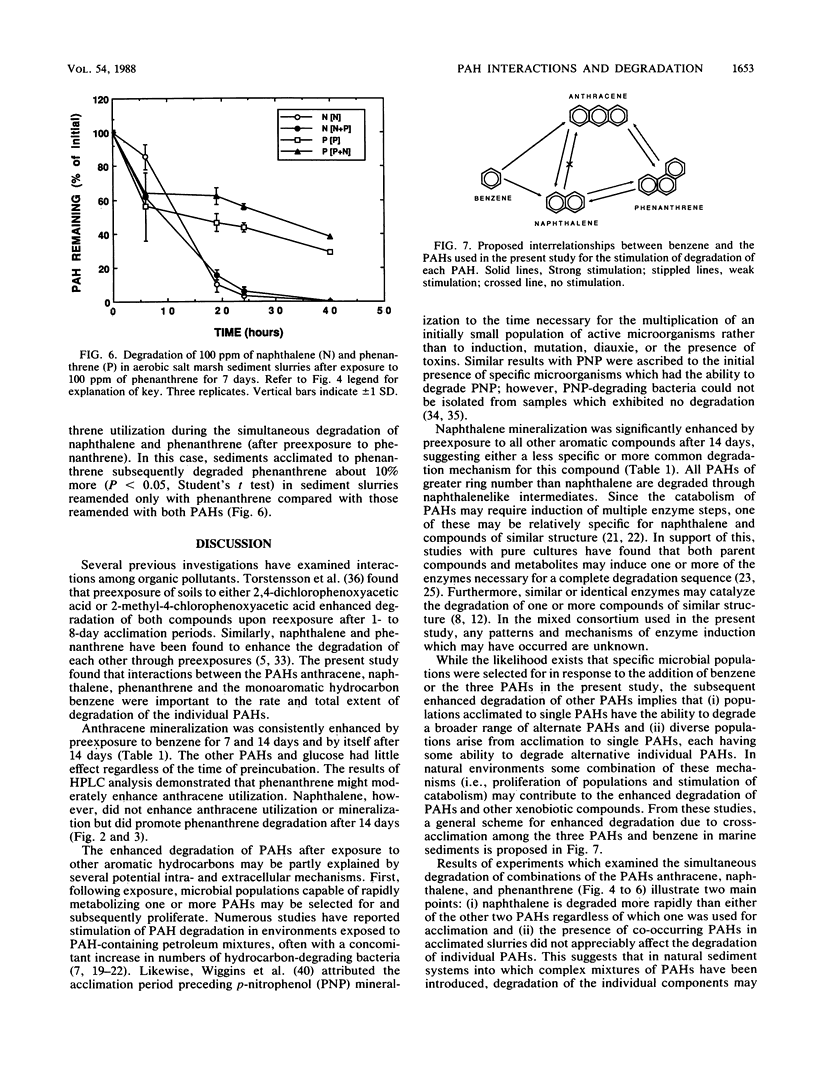
Rates of polycyclic aromatic hydrocarbon (PAH) degradation and mineralization were influenced by preexposure to alternate PAHs and a monoaromatic hydrocarbon at relatively high (100 ppm) concentrations in organic-rich aerobic marine sediments. Prior exposure to three PAHs and benzene resulted in enhanced [14C]naphthalene mineralization, while [14C]anthracene mineralization was stimulated only by benzene and anthracene preexposure. Preexposure of sediment slurries to phenanthrene stimulated the initial degradation of anthracene. Prior exposure to naphthalene stimulated the initial degradation of phenanthrene but had no effect on either the initial degradation or mineralization of anthracene. For those compounds which stimulated [14C]anthracene or [14C]naphthalene mineralization, longer preexposures (2 weeks) to alternative aromatic hydrocarbons resulted in an even greater stimulation response. Enrichment with individual PAHs followed by subsequent incubation with one or two PAHs showed no alteration in degradation patterns due to the simultaneous presence of PAHs. The evidence suggests that exposure of marine sediments to a particular PAH or benzene results in the enhanced ability of these sediments to subsequently degrade that PAH as well as certain other PAHs. The enhanced degradation of a particular PAH after sediments have been exposed to it may result from the selection and proliferation of specific microbial populations capable of degrading it. The enhanced degradation of other PAHs after exposure to a single PAH suggests that the populations selected have either broad specificity for PAHs, common pathways of PAH degradation, or both.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atlas R. M. Microbial degradation of petroleum hydrocarbons: an environmental perspective. Microbiol Rev. 1981 Mar;45(1):180–209. doi: 10.1128/mr.45.1.180-209.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnsley E. A. Bacterial oxidation of naphthalene and phenanthrene. J Bacteriol. 1983 Feb;153(2):1069–1071. doi: 10.1128/jb.153.2.1069-1071.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer J. E., Capone D. G. Degradation and mineralization of the polycyclic aromatic hydrocarbons anthracene and naphthalene in intertidal marine sediments. Appl Environ Microbiol. 1985 Jul;50(1):81–90. doi: 10.1128/aem.50.1.81-90.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer J. E., Capone D. G. Effects of four aromatic organic pollutants on microbial glucose metabolism and thymidine incorporation in marine sediments. Appl Environ Microbiol. 1985 Apr;49(4):828–835. doi: 10.1128/aem.49.4.828-835.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayly R. C., Wigmore G. J. Metabolism of phenol and cresols by mutants of Pseudomonas putida. J Bacteriol. 1973 Mar;113(3):1112–1120. doi: 10.1128/jb.113.3.1112-1120.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder J. A., Lader J. H. Effect of dissolved aromatic hydrocarbons on the growth of marine bacteria in batch culture. Appl Environ Microbiol. 1976 Jul;32(1):95–101. doi: 10.1128/aem.32.1.95-101.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. I., Evans W. C. Oxidative metabolism of naphthalene by soil pseudomonads. The ring-fission mechanism. Biochem J. 1964 May;91(2):251–261. doi: 10.1042/bj0910251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS W. C., FERNLEY H. N., GRIFFITHS E. OXIDATIVE METABOLISM OF PHENANTHRENE AND ANTHRACENE BY SOIL PSEUDOMONADS. THE RING-FISSION MECHANISM. Biochem J. 1965 Jun;95:819–831. doi: 10.1042/bj0950819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensley B. D., Gibson D. T., Laborde A. L. Oxidation of naphthalene by a multicomponent enzyme system from Pseudomonas sp. strain NCIB 9816. J Bacteriol. 1982 Mar;149(3):948–954. doi: 10.1128/jb.149.3.948-954.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambrick G. A., Delaune R. D., Patrick W. H. Effect of Estuarine Sediment pH and Oxidation-Reduction Potential on Microbial Hydrocarbon Degradation. Appl Environ Microbiol. 1980 Aug;40(2):365–369. doi: 10.1128/aem.40.2.365-369.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiman A. S., Cooper W. T. Solid-state 13C nuclear magnetic resonance spectroscopy of simultaneously metabolized acetate and phenol in a soil Pseudomonas sp. Appl Environ Microbiol. 1987 Jan;53(1):156–162. doi: 10.1128/aem.53.1.156-162.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitkamp M. A., Freeman J. P., Cerniglia C. E. Naphthalene biodegradation in environmental microcosms: estimates of degradation rates and characterization of metabolites. Appl Environ Microbiol. 1987 Jan;53(1):129–136. doi: 10.1128/aem.53.1.129-136.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbes S. E. Rates of microbial transformation of polycyclic aromatic hydrocarbons in water and sediments in the vicinity of a coal-coking wastewater discharge. Appl Environ Microbiol. 1981 Jan;41(1):20–28. doi: 10.1128/aem.41.1.20-28.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbes S. E., Schwall L. R. Microbial transformation of polycyclic aromatic hydrocarbons in pristine and petroleum-contaminated sediments. Appl Environ Microbiol. 1978 Feb;35(2):306–316. doi: 10.1128/aem.35.2.306-316.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes E. J., Bayly R. C. Control of catechol meta-cleavage pathway in Alcaligenes eutrophus. J Bacteriol. 1983 Jun;154(3):1363–1370. doi: 10.1128/jb.154.3.1363-1370.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B. F., Stanier R. Y. Regulation of the -ketoadipate pathway in Alcaligenes eutrophus. J Bacteriol. 1971 Aug;107(2):476–485. doi: 10.1128/jb.107.2.476-485.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPat-Polasko L. T., McCarty P. L., Zehnder A. J. Secondary substrate utilization of methylene chloride by an isolated strain of Pseudomonas sp. Appl Environ Microbiol. 1984 Apr;47(4):825–830. doi: 10.1128/aem.47.4.825-830.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGOFF M. H. Chemistry of oxidation of polycyclic aromatic hydrocarbons by soil pseudomonads. J Bacteriol. 1962 May;83:998–1004. doi: 10.1128/jb.83.5.998-1004.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamsuzzaman K. M., Barnsley E. A. The regulation of naphthalene metabolism in pseudomonads. Biochem Biophys Res Commun. 1974 Sep 23;60(2):582–589. doi: 10.1016/0006-291x(74)90280-0. [DOI] [PubMed] [Google Scholar]
- Spain J. C., Pritchard P. H., Bourquin A. W. Effects of adaptation on biodegradation rates in sediment/water cores from estuarine and freshwater environments. Appl Environ Microbiol. 1980 Oct;40(4):726–734. doi: 10.1128/aem.40.4.726-734.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain J. C., Van Veld P. A. Adaptation of natural microbial communities to degradation of xenobiotic compounds: effects of concentration, exposure time, inoculum, and chemical structure. Appl Environ Microbiol. 1983 Feb;45(2):428–435. doi: 10.1128/aem.45.2.428-435.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. D., Colwell R. R., Petrakis L. Biodegradation of petroleum by Chesapeake Bay sediment bacteria. Can J Microbiol. 1976 Mar;22(3):423–428. doi: 10.1139/m76-063. [DOI] [PubMed] [Google Scholar]
- Ward D. M., Brock T. D. Hydrocarbon biodegradation in hypersaline environments. Appl Environ Microbiol. 1978 Feb;35(2):353–359. doi: 10.1128/aem.35.2.353-359.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins B. A., Jones S. H., Alexander M. Explanations for the acclimation period preceding the mineralization of organic chemicals in aquatic environments. Appl Environ Microbiol. 1987 Apr;53(4):791–796. doi: 10.1128/aem.53.4.791-796.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


