Abstract
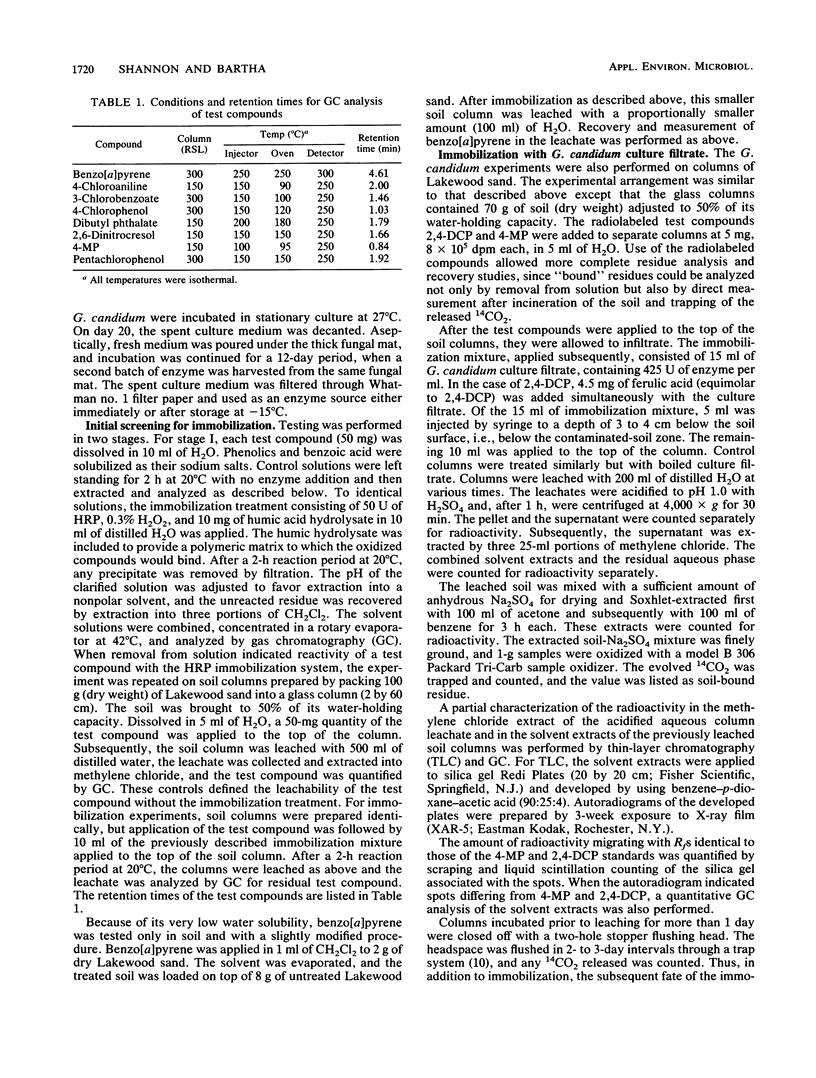
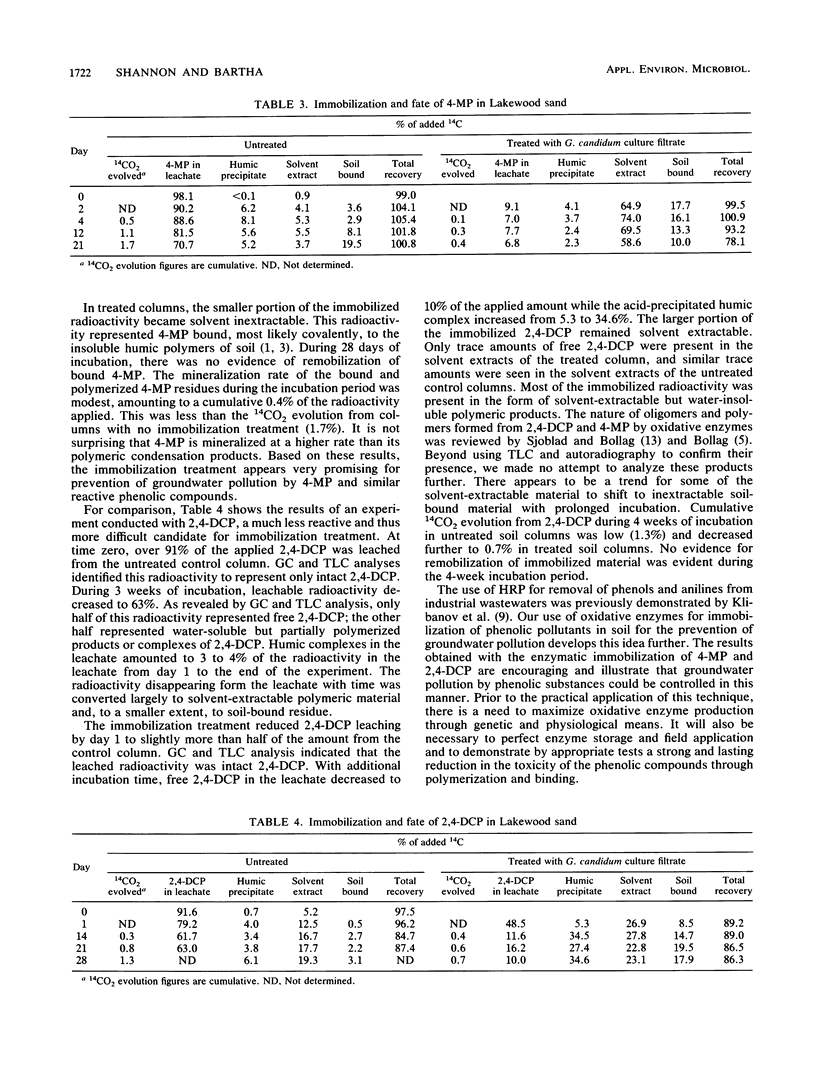
Screening of leachable toxic chemicals in a horseradish peroxidase-H2O2 immobilization system established that immobilization was promising for most phenolic pollutants but not for benzoic acid, 2,6-dinitrocresol, or dibutyl phthalate. The treatment did not mobilize inherently nonmobile pollutants such as anilines and benzo[a]pyrene. In a separate study, an extracellular laccase in the culture filtrate of Geotrichum candidum was selected from five fungal enzymes evaluated as a cost-effective substitute for horseradish peroxidase. This enzyme was used in demonstrating the immobilization and subsequent fate of 14C-labeled 4-methylphenol and 2,4-dichlorophenol in soil columns. When applied to Lakewood sand, 98.1% of 4-methylphenol was leached through with distilled water. Two days after immobilization treatment with the G. candidum culture filtrate, only 9.1% of the added 4-methylphenol was leached with the same volume of water. Of the more refractory test pollutant 2,4-dichlorophenol, 91.6% had leached at time zero and 48.5% had leached 1 day after the immobilization treatment. However, 2 weeks after immobilization, only 12.0% of the 2,4-dichlorophenol was leached compared with 61.7% from the control column that received no immobilization treatment. No remobilization of the bound pollutants was detected during 3- and 4-week incubation periods. Enzymatic immobilization of phenolic contaminants in soil appears to be a promising technique for the reduction of groundwater pollution by such substances.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bordeleau L. M., Bartha R. Biochemical transformations of herbicide-derived anilines in culture medium and in soil. Can J Microbiol. 1972 Dec;18(12):1857–1864. doi: 10.1139/m72-290. [DOI] [PubMed] [Google Scholar]
- Klibanov A. M., Tu T. M., Scott K. P. Peroxidase-catalyzed removal of phenols from coal-conversion waste waters. Science. 1983 Jul 15;221(4607):259–261. doi: 10.1126/science.221.4607.259-a. [DOI] [PubMed] [Google Scholar]
- Marinucci A. C., Bartha R. Apparatus for monitoring the mineralization of volatile C-labeled compounds. Appl Environ Microbiol. 1979 Nov;38(5):1020–1022. doi: 10.1128/aem.38.5.1020-1022.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pye V. I., Patrick R. Ground water contamination in the United States. Science. 1983 Aug 19;221(4612):713–718. doi: 10.1126/science.6879171. [DOI] [PubMed] [Google Scholar]


