Abstract
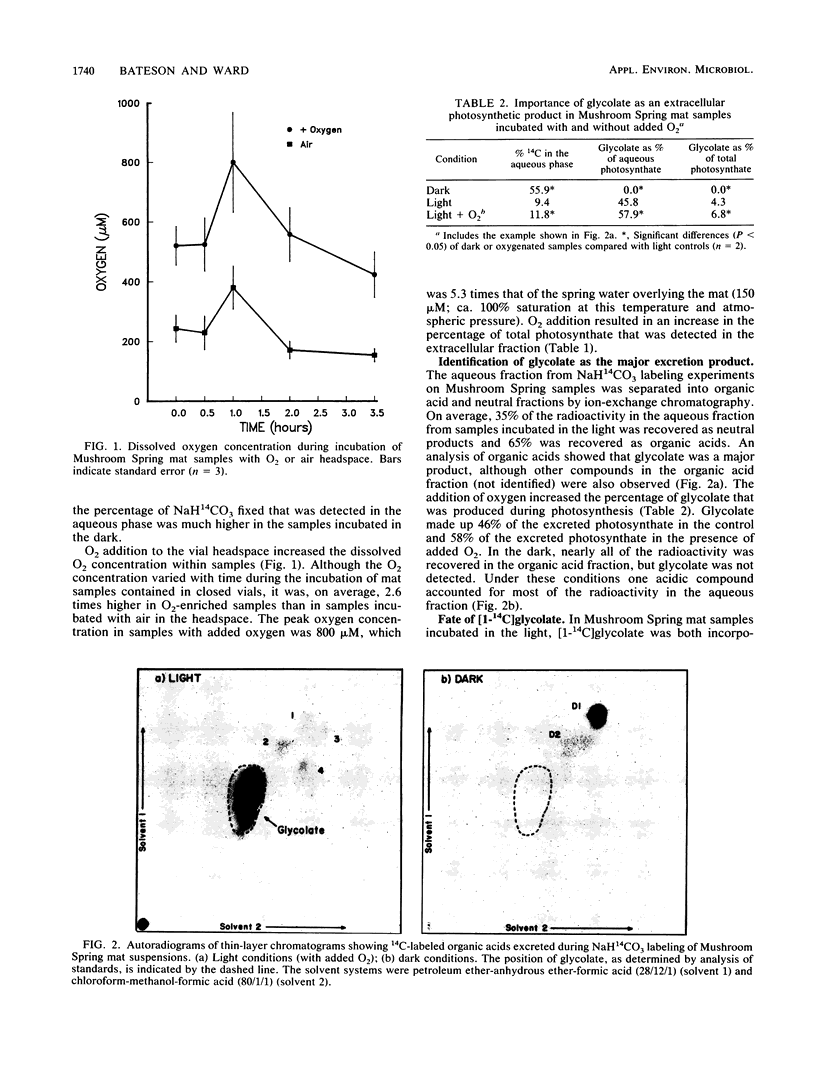
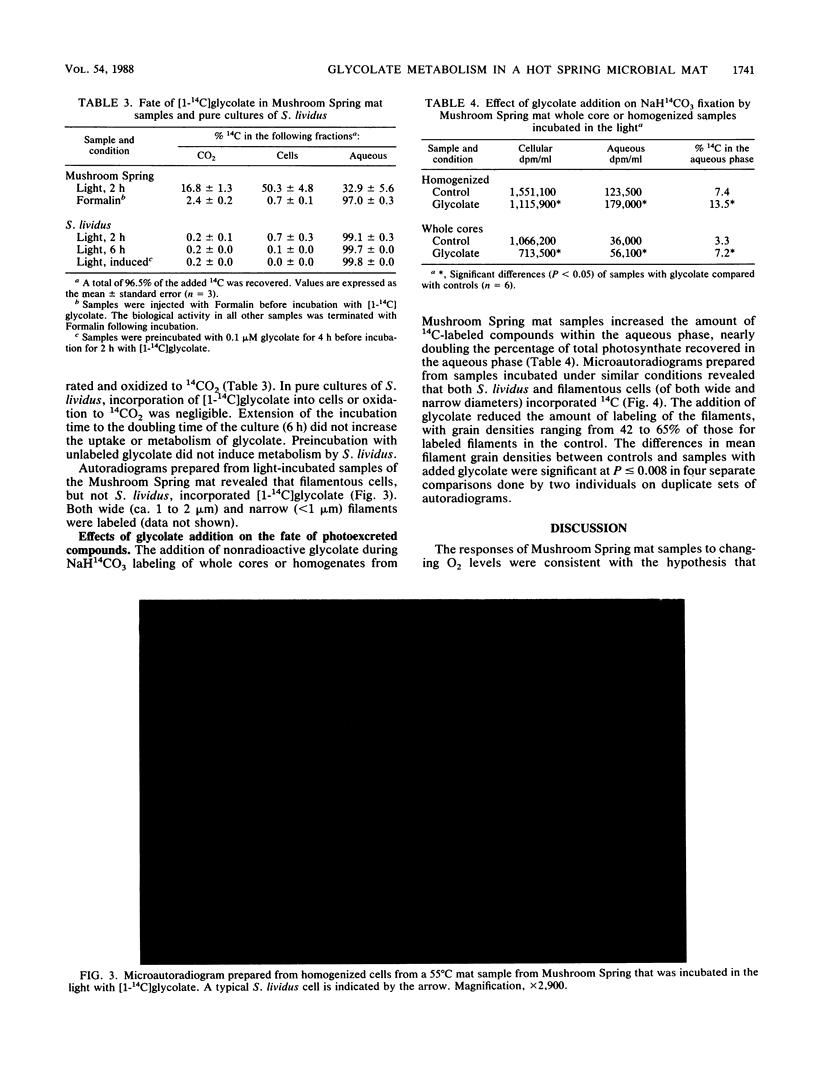
Photosynthesis by Synechococcus lividus, the sole oxygenic phototroph inhabiting the surface of the 55°C cyanobacterial mat in Mushroom Spring, Yellowstone National Park, causes superoxic and alkaline conditions which promote glycolate photoexcretion. At O2 concentrations characteristic of the top 2 mm of mat during the day, up to 11.8% of NaH14CO3 fixed in the light was excreted, and glycolate accounted for up to 58% of the excreted photosynthate. Glycolate was neither incorporated nor metabolized by S. lividus, but it was incorporated by filamentous microorganisms in the mat. Incubation of mat samples with NaH14CO3 resulted in labeling of both S. lividus and filaments, but the addition of nonradioactive glycolate increased the level of 14C in the aqueous phase and decreased the extent of labeling of filaments. This suggests that cross-feeding of glycolate from S. lividus to filamentous heterotrophs occurs and that underestimation of the extent of photoexcretion is probable.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. L., Tayne T. A., Ward D. M. Formation and fate of fermentation products in hot spring cyanobacterial mats. Appl Environ Microbiol. 1987 Oct;53(10):2343–2352. doi: 10.1128/aem.53.10.2343-2352.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleiweis A. S., Reeves H. C., Ajl S. J. Rapid separation of some common intermediates of microbial metabolism by thin-layer chromatography. Anal Biochem. 1967 Aug;20(2):335–338. doi: 10.1016/0003-2697(67)90039-5. [DOI] [PubMed] [Google Scholar]
- Castenholz R. W. Thermophilic blue-green algae and the thermal environment. Bacteriol Rev. 1969 Dec;33(4):476–504. doi: 10.1128/br.33.4.476-504.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codd G. A., Smith B. M. Glycollate formation and excretion by the purple photosynthetic bacterium Rhodospirillum rubrum. FEBS Lett. 1974 Nov 1;48(1):105–108. doi: 10.1016/0014-5793(74)81073-2. [DOI] [PubMed] [Google Scholar]
- Eickenbusch J. D., Beck E. Evidence for involvement of 2 types of reaction in glycolate formation during photosynthesis in isolated spinach chloroplasts. FEBS Lett. 1973 Apr 15;31(2):225–228. doi: 10.1016/0014-5793(73)80109-7. [DOI] [PubMed] [Google Scholar]
- Kallas T., Castenholz R. W. Internal pH and ATP-ADP pools in the cyanobacterium Synechococcus sp. during exposure to growth-inhibiting low pH. J Bacteriol. 1982 Jan;149(1):229–236. doi: 10.1128/jb.149.1.229-236.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revsbech N. P., Ward D. M. Microelectrode studies of interstitial water chemistry and photosynthetic activity in a hot spring microbial mat. Appl Environ Microbiol. 1984 Aug;48(2):270–275. doi: 10.1128/aem.48.2.270-275.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandbeck K. A., Ward D. M. Fate of immediate methane precursors in low-sulfate, hot-spring algal-bacterial mats. Appl Environ Microbiol. 1981 Mar;41(3):775–782. doi: 10.1128/aem.41.3.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandbeck K. A., Ward D. M. Temperature adaptations in the terminal processes of anaerobic decomposition of yellowstone national park and icelandic hot spring microbial mats. Appl Environ Microbiol. 1982 Oct;44(4):844–851. doi: 10.1128/aem.44.4.844-851.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOLBERT N. E., ZILL L. P. Excretion of glycolic acid by algae during photosynthesis. J Biol Chem. 1956 Oct;222(2):895–906. [PubMed] [Google Scholar]
- Tayne T. A., Cutler J. E., Ward D. M. Use of chloroflexus-specific antiserum to evaluate filamentous bacteria of a hot spring microbial mat. Appl Environ Microbiol. 1987 Aug;53(8):1962–1964. doi: 10.1128/aem.53.8.1962-1964.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward D. M., Olson G. J. Terminal processes in the anaerobic degradation of an algal-bacterial mat in a high-sulfate hot spring. Appl Environ Microbiol. 1980 Jul;40(1):67–74. doi: 10.1128/aem.40.1.67-74.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward D. M. Thermophilic methanogenesis in a hot-spring algal-bacterial mat (71 to 30 degrees C). Appl Environ Microbiol. 1978 Jun;35(6):1019–1026. doi: 10.1128/aem.35.6.1019-1026.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G. Metabolism of one-carbon compounds by chemotrophic anaerobes. Adv Microb Physiol. 1983;24:215–299. doi: 10.1016/s0065-2911(08)60387-2. [DOI] [PubMed] [Google Scholar]