Abstract
4-Coumarate:CoA ligases (4CLs, EC 6.2.1.12) are a group of enzymes necessary for maintaining a continuous metabolic flux for the biosynthesis of plant phenylpropanoids, such as lignin and flavonoids, that are essential to the survival of plants. So far, various biochemical and molecular studies of plant 4CLs seem to suggest that 4CL isoforms in plants are functionally indistinguishable in mediating the biosynthesis of these phenolics. However, we have discovered two functionally and structurally distinct 4CL genes, Pt4CL1 and Pt4CL2 (63% protein sequence identity), that are differentially expressed in aspen (Populus tremuloides). The Escherichia coli-expressed and purified Pt4CL1 and Pt4CL2 proteins exhibited highly divergent substrate preference as well as specificity that reveal the association of Pt4CL1 with the biosynthesis of guaiacyl–syringyl lignin and the involvement of Pt4CL2 with other phenylpropanoid formation. Northern hybridization analysis demonstrated that Pt4CL1 mRNA is specifically expressed in lignifying xylem tissues and Pt4CL2 mRNA is specifically expressed in epidermal layers in the stem and the leaf, consistent with the promoter activities of Pt4CL1 and Pt4CL2 genes based on the heterologous promoter-β-glucouronidase fusion analysis. Thus, the expression of Pt4CL1 and Pt4CL2 genes is compartmentalized to regulate the differential formation of phenylpropanoids that confer different physiological functions in aspen; Pt4CL1 is devoted to lignin biosynthesis in developing xylem tissues, whereas Pt4CL2 is involved in the biosynthesis of other phenolics, such as flavonoids, in epidermal cells.
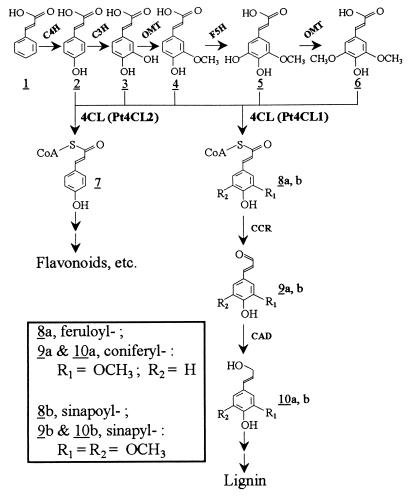
Plant secondary metabolites confer various physiological functions for plants to survive and to adapt to environmental perturbations. These metabolites are largely phenylpropanoids converted from cinnamic acid (Fig. 1, 1) and its hydroxylated derivatives (Fig. 1, 2-6) through pathways mediated by various enzymes. The 4-Coumarate:CoA ligases (4CLs, EC 6.2.1.12) are a group of essential enzymes in these pathways, as they catalyze the necessary activation of the hydroxylated cinnamic acids (Fig. 1, 2-6) to their corresponding thioesters (Fig. 1, 7 and 8 a and b) (1), thereby maintaining a continuous metabolic flux for the biosynthesis of phenylpropanoids, such as flavonoids and lignin (2–4).
Figure 1.
Biosynthetic pathways of hydroxylated cinnamic acids and cinnamoyl CoA thioesters for the formation of lignin and other phenylpropanoids. C4H, cinnamate 4-hydroxylase; C3H, 4-coumarate 3-hydroxylase; OMT, O-methyltransferase; F5H, ferulate 5-hydroxylase; Pt4CL1 and 2, P. tremuloides 4CL1 and 2; CCR, cinnamoyl-CoA reductase; CAD, cinnamyl alcohol dehydrogenase.
In accordance with the different physiological functions of flavonoids and lignin, distinct 4CL isoenzyme activities in mediating the biosynthesis of these two groups of phenolic metabolites have been detected in soybean cell cultures (2, 3), suggesting the expression of divergent genes encoding functionally distinct 4CL isoforms. So far, cDNAs and genomic DNAs encoding 4CL have been cloned from various plant species (for reviews, see refs. 5 and 6), and it was concluded (5) that, among these species, only tobacco (5) and soybean (7) contain divergent 4CL gene members. However, the two tobacco 4CL genes (4CL1 and 4CL2), exhibiting high protein sequence homology (81% identity/89% similarity), were not functionally divergent, as the recombinant 4CL1 and 4CL2 proteins displayed essentially identical substrate specificities (5). The expression patterns of these two 4CL genes in various tobacco organs were also indistinguishable, further demonstrating the duplicating role of these two genes in tobacco. On the other hand, the two soybean 4CL genes (GM4CL14 and GM4CL16) were differentially regulated in response to both pathogen attack of soybean roots and elicitor treatment of soybean cell cultures. The pathogen- or elicitor-inducible GM4CL16 gene was speculated to encode the 4CL isoform that mediates the formation of flavonoids (7). However, with only a partial sequence, the exact biochemical functions of the proteins encoded by these two soybean 4CL genes are unknown. Thus, the molecular and biochemical evidence of the existence of functionally distinct 4CL isoforms is still lacking to provide a better understanding of the nature of 4CL isoforms in directing the metabolic flux for the biosynthesis of different classes of phenolics with specialized functions in plants.
We report here the existence of two structurally and functionally distinct 4CL genes, Pt4CL1 and Pt4CL2, in aspen (P. tremuloides). Most importantly, these two genes are expressed in a compartmentalized manner. Biochemical and in vivo expression and promoter activity analyses provided strong evidence that Pt4CL1 is associated with lignin biosynthesis in developing xylem of woody stems, whereas Pt4CL2 takes part in the biosynthesis of phenylpropanoids other than lignin in epidermal cells of the stem and the leaf. The discovery of compartmentalized expression of two structurally and functionally divergent 4CL genes may help answer the enduring question of how 4CL differentially regulates the biosynthesis of phenylpropanoids that have distinct physiological functions in trees and may provide new insights into the biotechnological manipulation of the expression of these two genes to engineer desirable transgenic tree species.
MATERIALS AND METHODS
Plant Material.
Developing secondary xylem tissues were collected from 4-year-old quaking aspen (P. tremuloides Michx.) grown on the campus of Michigan Technological University and young leaves and internodes from greenhouse-grown quaking aspen. Tissues were immediately frozen and stored in liquid nitrogen until used for protein, RNA, and DNA isolation.
Cloning of Pt4CL1 cDNA and Gene.
Total RNA was isolated from developing xylem according to Bugos et al. (8), from which mRNA was isolated using the Poly(A)+ mRNA isolation kit (Tel-Test, Friendswood, TX). A unidirectional λ gt22 expression cDNA library was constructed from xylem mRNA using Superscript λ System (Life Technologies, Rockville, MD) and Gigapack Packaging Extracts (Stratagene) (9). A 32P-labeled parsley 4CL-2 cDNA (a gift from Dr. Carl Douglas, University of British Columbia) probe was used to screen 5 × 105 plaque-forming units of the aspen xylem cDNA library. Three positive clones were obtained after three rounds of plaque purification and were end-sequenced using the Δ Taq Cycle Sequencing Kit (Amersham). Because end-sequencing revealed that these three clones were analogous to each other, only two of them with longer inserts were completely sequenced for both strands and found to be identical full-length cDNAs and were designated as Pt4CL1.
The full-length Pt4CL1 cDNA was then used for screening an aspen genomic library constructed by cloning Sau3A I partial-digested and sucrose gradient-selected genomic DNA fragments into the BamHI site of λDASH II vector (CLONTECH). Eleven positive clones were obtained and subjected to further purification. Four clones were found to contain the full coding sequence with at least a 2-kilobase (kb) 5′ flanking region and one of which, Pt4CL1 g-4 (≈15 kb), was selected for restriction mapping and partial sequencing to confirm its authenticity. The 5′ flanking region then was subcloned into pGEM 7Z for further characterization.
Cloning of Pt4CL2 cDNA and Gene.
Two sense and one antisense degenerate primers (Fig. 2) were designed based on the consensus regions of all known plant 4CL amino acid sequences. Two sense primers included R1S (5′-TTGGATCCGGIACIACIGGIYTICCIAARGG), which is located at the first putative AMP-binding domain and H1S (5′-TTGGATCCGTIGCICARCARGTIGAYGG), which is located at 9-aa downstream from the first AMP-binding domain. The antisense primer R2A (5′-ATGTCGACCICKDATRCADATYTCICC) was designed based on the second putative AMP-binding domain, the CGEICIRG motif. Ten micrograms of total RNA each isolated from top (1st–4th) and lower (6th–10th) aspen stem internodes, respectively, were reverse transcribed, and one-fifth of the reaction mixtures were used as the template for PCR amplification with 2 μM each oligo-dT20 primer and R1S primer, 200 μM dNTPs, and 2.5 units of Taq DNA polymerase (Promega) in a 50 μl reaction. Two microliters of each reverse transcription–PCR (RT-PCR) reaction products were subjected to a nested–PCR using H1S and R2A as the primers. Conditions for both runs of PCR were: 94°C/5 min, 30 cycles of 94°C/45 sec, 50°C/1 min, 72°C/1 min 30 sec, and 72°C/5 min. A ≈600-bp fragment amplified from top internode RNA was cloned (Pt4CL2–600) by using the TA cloning kit (Invitrogen) and was used to screen the aspen genomic library as described above, and seven positive clones were identified. One of the clones, Pt4CL2 g-11 (≈13 kb), was selected, subcloned, and sequenced for its 5′ flanking and full coding regions. Based on the genomic sequence, two primers (2A: 5′-TCTGTCTAGATGATGTCGTGGCCACGG and 2B: 5′-TTAGATCTCTAGGACATGGTGGTGGC) were designed around the deduced translation start and stop sites (Fig. 2) of Pt4CL2 g-11, respectively, and used for RT-PCR amplification of top internode RNA as described above. A 1.7-kb cDNA fragment was amplified, cloned, sequenced, and designated as Pt4CL2.
Figure 2.
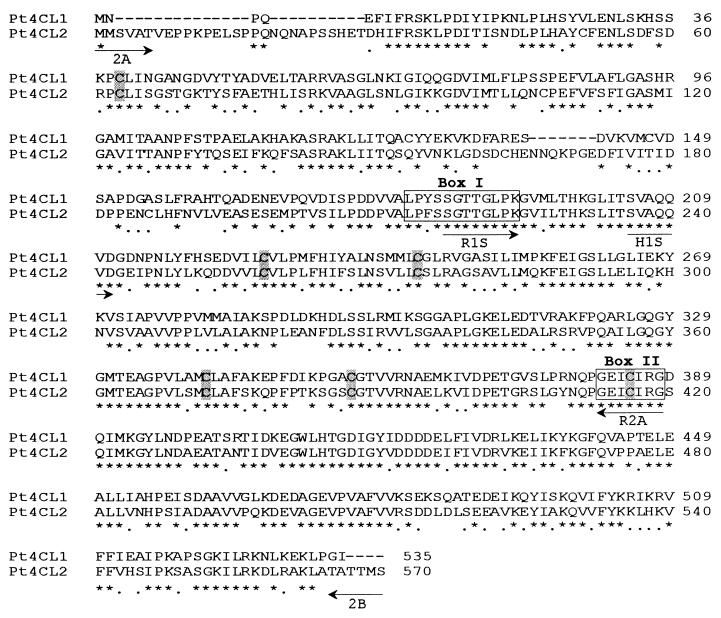
Alignment of the deduced amino acid sequences of aspen Pt4CL1 (GenBank accession no. AF041049) and Pt4CL2 (GenBank accession no. AF041050). Symbols denote identical (*) and similar (•) amino acids and conserved Cys residues (shaded). Boxes I and II are the two putative AMP-binding motifs (19, 20). Locations of primers used for RT-PCR are indicated by arrows. Sequence alignment was performed using the clustlaw (1.60) program through the European Molecular Biology Laboratory world wide web server.
Expression of Recombinant Pt4CL1 and Pt4CL2 in E. coli and Purification of Recombinant Proteins.
PCR was used to introduce a BamHI site at the 5′ and 3′ ends of Pt4CL1 cDNA, and the amplified product was digested with BamHI and cloned into the same site of pQE-31 containing a histidine tag (6xHis tag, Qiagen, Chatsworth, CA). Similarly, a BglII site was introduced into the 5′ end of Pt4CL2 cDNA, and a 400-bp PCR fragment was amplified, sequenced to confirm the fidelity of PCR, and used to replace the corresponding 5′ end of the original Pt4CL2 cDNA. The engineered Pt4CL2 cDNA was cloned into the 6xHis-containing pQE-32 vector (Qiagen) at BamHI and KpnI sites. The expression vector was transferred into E. coli strain M15, and the growth and induction of bacterial cells with isopropyl β-d-thiogalactoside were performed according to the manufacturer’s protocol (Qiagen). The isolation and purification of the Pt4CL1- and Pt4CL2–6xHis tag fusion proteins were conducted using His⋅Bind Resin (Novagen) as described by Li et al. (10).
Biochemical and Molecular Analysis.
Plant crude protein extracted from aspen developing xylem tissues (11) and purified Pt4CL1 and Pt4CL2 recombinant proteins were used for enzyme assays as described (12). The 5-hydroxyferulic acid was synthesized (10), and all of the other substrates were obtained from Sigma. Protein concentrations were determined by the Bradford assay (13) using BSA as a standard. Southern and Northern blot analyses using Pt4CL1 or Pt4CL2 cDNA as a probe were performed as described (11).
Preparation of Promoter-β-Glucouronidase (GUS) Fusion Constructs and Transformation of Tobacco.
A 1-kb Pt4CL1 gene promoter fragment containing a 17-bp coding sequence was subcloned into pGEM7Z (Promega) at SphI and EcoRI sites. A BamHI site was then introduced in front of the translation start site by PCR, and the engineered 1-kb SphI-BamHI promoter fragment was subcloned into pNoTA (5 prime→3 prime), sequenced, and released again by HindIII and BamHI digestion to clone the fragment into pBI101 (CLONTECH). The resulting Pt4CL1 gene promoter-GUS fusion binary vector was named Pt4CL1p-GUS. For the construction of Pt4CL2 gene promoter-GUS fusion binary vector, a 1.5-kb Pt4CL2 gene promoter fragment containing a 300-bp coding sequence was subcloned into pBluescipt II SK+ (Stratagene) at HindIII and EcoRI sites. A similar strategy to the above was used to engineer a BamHI site in front of the Pt4CL2 translation start site, and the 1.2-kb HindIII-BamHI promoter fragment was cloned into pBI101 to create the Pt4CL2 gene promoter-GUS fusion construct (Pt4CL2p-GUS). The plasmids were mobilized into Agrobacterium tumefaciens strain C58/pMP90 (14) by the freeze and thaw method (15). Leaf disc transformation of tobacco (Nicotiana tabacum Hanana) was conducted as described (16). GUS activity of transgenic tobacco was analyzed histochemically according to Tsai et al. (17).
RESULTS AND DISCUSSION
Cloning and Characterization of Aspen Pt4CL1 and Pt4CL2 cDNAs.
We are interested in differentially expressed 4CL genes and their biochemical roles in aspen. In a separate study to characterize lignification in aspen stems, we noticed that the lignin composition in the top four internodes (referred to as top internodes hereafter) was different from that in the fifth and subsequent internodes (unpublished results), suggesting the involvement of developmentally regulated differential expression of lignin pathway genes during the transition from primary to secondary growth in aspen stem. To investigate whether this transition regulates differential expression of 4CL gene members, we focused on cloning 4CL genes from top and lower (6th–10th) internodes and secondary-developing xylem tissue of aspen stems. We first used parsley 4CL cDNA (18) as a probe to screen an aspen xylem cDNA library and isolated a 1,915-bp cDNA designated as Pt4CL1, which has an ORF encoding a 535-aa protein (Fig. 2) with a calculated Mr of 58,498 and a pI of 5.9. The deduced Pt4CL1 protein exhibited 57–76% overall sequence identity (75–88% similarity) to all of the other known full-length plant 4CL proteins (a total of 14 available in the database). Preliminary Northern hybridization analysis indicated a high level of Pt4CL1 mRNA in aspen developing xylem tissue. Next, we used RT-PCR coupled with nested–PCR-based cloning to isolate other possible 4CL gene members from RNAs isolated from top and lower internodes, respectively. Oligo-deoxythymidine (dT) and a degenerate primer R1S (Fig. 2) based on the protein sequence of a highly conserved AMP-binding domain found in a number of plant ATP-dependent enzymes (19) were used for RT-PCR and a set of degenerate primers (H1S and R2A, Fig. 2) for nested–PCR. cDNA fragments ≈600 bp in length were isolated from both top and lower internode RNA and cloned. Nucleotide sequence analysis revealed that clones derived from lower internodes were identical to Pt4CL1, whereas clones isolated from top internodes could be divided into two groups (T1 and T2). Clones in group T1 were found identical to Pt4CL1. Clones in group T2 shared 60–75% sequence homology with other plant 4CL genes but were distinct from Pt4CL1 cDNA and designated as Pt4CL2–600. These results together with preliminary Northern hybridization analysis suggested that Pt4CL2–600 represents a fragment of another aspen 4CL gene expressed in top internodes.
Using Pt4CL2–600 cDNA as a probe to screen the aspen genomic library, one clone, Pt4CL2 g-11 (≈13 kb), was identified to include a full-length coding sequence and a 1.8-kb 5′ flanking region. A pair of primers (2A and 2B, Fig. 2) based on the sequences around the translation start and stop codons in Pt4CL2 g-11, respectively, was used in RT-PCR to amplify cDNAs from RNA isolated from aspen top internodes. A 1.7-kb cDNA fragment was cloned, sequenced, and confirmed to be identical to the coding sequence of Pt4CL2 g-11. This full-length cDNA, designated as Pt4CL2, encodes a 570-aa protein (Fig. 2) with a calculated Mr of 61,831 and a pI of 5.1. The deduced Pt4CL2 protein shares 63% sequence identity (72% similarity) with Pt4CL1 protein and shows 58–72% overall identity (77–83% similarity) to all of the other known full-length plant 4CLs. A low sequence conservation (48% identity/54% similarity) between Pt4CL1 and Pt4CL2 proteins was observed in a stretch of 180 aa at the N terminus. However, a high homology (69% identity/80% similarity) between these two proteins was found in the rest of the amino acid sequences where two conserved putative AMP-binding signatures were located. The first signature (box I in Fig. 2) consists of a STG (serine/threonine/glycine)-rich domain followed by a PKG (proline/lysine/glycine) triplet (19) and the second a “GEICIRG” motif (box II) (20). Six conserved cysteine residues commonly found in plant 4CLs (7) also were found in Pt4CL1 and Pt4CL2 proteins (Fig. 2). Based on the multiple sequence alignment, a unique sequence feature was noticed for Pt4CL2 protein in that it has a ≈20-aa longer N-terminal sequence when compared with all of the other plant 4CLs except Lithospermum 4CL (21) and rice 4CL (GenBank accession no. L43362). Phylogenetic tree analysis of all known plant 4CLs also showed that Pt4CL2 and Lithospermum and rice 4CLs are within the same cluster, whereas Pt4CL1 together with all of the other plant 4CLs form a different cluster (data not shown), further suggesting that Pt4CL1 and Pt4CL2 are encoded by two divergent genes.
Pt4CL1 and Pt4CL2 Genes Are Differentially Expressed and Have Distinct Biochemical Functions.
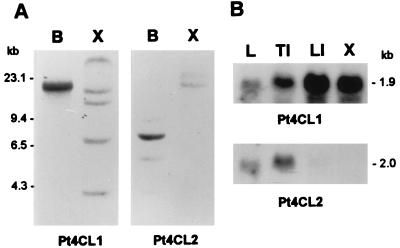
Southern blot analysis of aspen genomic DNA with full-length Pt4CL1 and Pt4CL2 cDNAs as probes, respectively, at high stringency revealed that each cDNA probe hybridized to multiple restricted DNA fragments with clearly distinguishable patterns, suggesting that these two cDNAs do not cross-hybridize to each other and that multiple members could be present in both Pt4CL1 and Pt4CL2 gene families (Fig. 3A). Northern blot analysis of aspen RNA showed high Pt4CL1 mRNA levels in secondary developing xylem and lower internodes, moderate level in top internodes, and low level in leaves, as indicated by the intensity of the hybridizing bands of ≈1.9 kb that corresponds to the length of the Pt4CL1 transcript (Fig. 3B). Because lignification is known to take place in secondary developing xylem and vascular tissues in leaves and young internodes, these Northern hybridization results are consistent with the interpretation that Pt4CL1 is associated with lignification. In addition, these Pt4CL1 gene expression patterns are in concert with those of other lignin pathway genes encoding C4H, OMT, and F5H (ref. 22 and unpublished results), providing additional evidence that Pt4CL1 is involved in lignification. In sharp contrast, no Pt4CL2 mRNA message could be detected in secondary developing xylem and the message was barely noticeable in lower internodes (Fig. 3B). However, a high level of Pt4CL2 mRNA was observed in top internodes and a weak expression of Pt4CL2 mRNA was found in leaves (Fig. 3B), suggesting that Pt4CL2 could be involved in phenylpropanoid biosynthesis in these green tissues, but the absence of Pt4CL2 mRNA from secondary developing xylem and lower internodes precludes the involvement of Pt4CL2 as an essential enzyme in lignin biosynthesis in woody xylem.
Figure 3.
(A) Southern blot analysis of aspen genomic DNA (10 μg per lane) digested with BamHI (lane B) and XbaI (lane X), probed with 32P-labeled Pt4CL1 and Pt4CL2 cDNAs, respectively. λ DNA digested with HindIII was used as size markers, and values are given in kb. (B) Northern blot analysis of total RNA (10 μg per lane) from young leaf (lane L), top internodes (TI), lower internodes (LI), and secondary developing xylem (X) of aspen, probed with 32P-labeled Pt4CL1 and Pt4CL2 cDNAs, respectively.
To compare the biochemical functions of Pt4CL1 and Pt4CL2, E. coli-expressed Pt4CL1 and Pt4CL2 proteins were purified to apparent homogeneity with a histidine tag sequence-specific affinity column (Novagen) (10), and their purity was demonstrated by a single major band of ≈60 kDa for Pt4CL1 and 63 kDa for Pt4CL2, as estimated by SDS/PAGE (data not shown). The purified recombinant proteins were characterized for their catalytic activities with various hydroxycinnamic acid derivatives (Table 1). The results showed that, in addition to a clear divergence in substrate preference between Pt4CL1 and Pt4CL2, the specific activities of Pt4CL1 with 4-coumaric (2 in Fig. 1), caffeic (3), and ferulic (4) acids were ≈6, 10, and 16 times, respectively, higher than those of Pt4CL2, reflecting the markedly different catalytic efficiency between these two aspen 4CLs. The most striking functional difference between Pt4CL1 and Pt4CL2 is that Pt4CL1 can efficiently use 5-hydroxyferulic acid (5 in Fig. 1), a substrate for the typical syringyl lignin in angiosperms (23–24), whereas Pt4CL2 is inactive with this compound (Table 1). Like Pt4CL1, aspen secondary xylem crude protein extracts also used 5-hydroxyferulic acid in addition to 4-coumaric, caffeic, and ferulic acids with relative specific activities that were comparable to the relative specific activities of the purified recombinant Pt4CL1 (Table 1), providing evidence that 4CL activities in aspen xylem extracts are derived mainly from Pt4CL1. These results are consistent with the presence of a high level of Pt4CL1 mRNA in and the absence of Pt4CL2 mRNA (Fig. 3B) from aspen developing xylem.
Table 1.
Substrate specificity of 4CL in aspen xylem crude extracts and of E. coli-expressed Pt4CL1 and Pt4CL2 recombinant proteins
| Substrate | Specific activity*, pkat/mg protein
|
||
|---|---|---|---|
| Aspen xylem extracts | Purified recombinant Pt4CL1 | Purified recombinant Pt4CL2 | |
| 4-Coumaric acid | 738.89 ± 19.25 | 11484.48 ± 128.30 | 1878.95 ± 41.40 |
| Caffeic acid | 250.00 ± 8.33 | 5944.44 ± 146.99 | 578.14 ± 15.65 |
| Ferulic acid | 411.67 ± 22.05 | 8222.22 ± 192.45 | 496.84 ± 15.65 |
| 5-Hydroxyferulic acid | 91.67 ± 14.43 | 2166.67 ± 146.99 | 0 |
Specific activities were mean ± SD (n = 3 independent assays).
The preference of Pt4CL1 for lignin-specific substrates, such as ferulic and 5-hydroxyferulic acids (Table 1), suggest that Pt4CL1 is involved in lignin biosynthesis. The lignin-related Pt4CL1 protein function is in agreement with the finding that Pt4CL1 mRNA is specifically expressed in lignifying xylem tissues. The fact that the absence of Pt4CL2 mRNA from lignifying xylem tissues and its presence in young green tissues (Fig. 3B), indicates the involvement of Pt4CL2 in converting its hydroxycinnamic acid substrates into phenylpropanoid pathways other than lignin (Fig. 1). In fact, in the context of substrate utilization, Pt4CL1 and Pt4CL2 resemble closely the lignin- and flavonoid-associated isoenzymes, respectively, detected in soybean cell cultures as described by Knoblock and Hahlbrock (2). We speculate that, because Pt4CL1 and Pt4CL2 proteins are highly divergent in their N-terminal sequences (amino acids 1–180 in Fig. 2), including a 24-aa shorter sequence in Pt4CL1, these N-terminal sequences might be involved in phenolic substrate binding specificity, contributing to the marked difference in catalytic function between these two proteins.
Although Pt4CL1 and aspen xylem protein extracts use 5-hydroxyferulic acid and Pt4CL2 does not, none of these three enzyme systems shows activity with sinapic acid, which generally is considered as a precursor of syringyl lignin in angiosperms. These results are consistent with the previous studies that 5-hydroxyferulic acid is an effective substrate for 4CLs in protein extracts of lignifying-developing xylem tissues from various angiosperm tree species, whereas sinapic acid is not (23–25). It has been further demonstrated that, without the participation of sinapic acid, sinapyl alcohol (Fig. 1, 10a) is formed sequentially from 4CL-activated 5-hydroxyferulic acid for making syringyl lignin, suggesting that 5-hydroxyferulic acid is a factual precursor for the biosynthesis of syringyl lignin in angiosperm trees (24, 25). Therefore, in aspen, Pt4CL1 is a key lignin-pathway enzyme that oversees a continuous metabolic flux for syringyl lignin formation by directing 5-hydroxyferulic acid into the network of lignin biosynthesis. The ability of Pt4CL1 in using multiple substrates in vitro implies that Pt4CL1 is potentially capable of mediating the biosynthesis of phenylpropanoids, including lignin. However, lignification rather than other phenylpropanoid biosynthesis predominates in developing xylem (4), suggesting that the involvement of Pt4CL1 in developing xylem is primarily with lignin biosynthesis. Thus, in aspen xylem, Pt4CL1 channels the appropriate hydroxycinnamic acid derivatives to the biosynthesis of lignin, including the typical angiosperm-associated syringyl moiety that could be derived from Pt4CL1-activated 5-hydroxyferulic acid.
Heterologous Pt4CL1 and Pt4CL2 Promoter-GUS Fusion Analyses Indicate that Pt4CL1 Gene Expression Is Xylem-Specific and Pt4CL2 Is Epidermis-Specific.
We isolated Pt4CL1 and Pt4CL2 genomic clones to further characterize their gene structures and promoter activities. The insignificant sequence similarity between the 5′- and 3′-noncoding regions of these two genes and their distinct exon–intron organizations (four introns in Pt4CL1 and five in Pt4CL2) further substantiate their functional and perhaps evolutionary divergency (data not shown). Striking differences also were observed in the promoter sequences of these two genes. Three cis-acting elements, box P (CCTTTCACCAACCCCC), box A (CCGTTC), and box L (TCTCACCAACC), previously shown to be consensus in all known plant phenylalanine ammonia-lyase (PAL) and 4CL gene promoters (26, 27), were identified within the 1-kb 5′ flanking sequence of Pt4CL1 (GenBank accession no. AF041051). However, none of these boxes could be found within the analyzed 1.2-kb 5′ flanking region of Pt4CL2 (GenBank accession no. AF041052), suggesting that promoter differences between Pt4CL1 and Pt4CL2 genes could be responsible for the strikingly different patterns of tissue-specific expression of these genes, as observed in the Northern analysis.
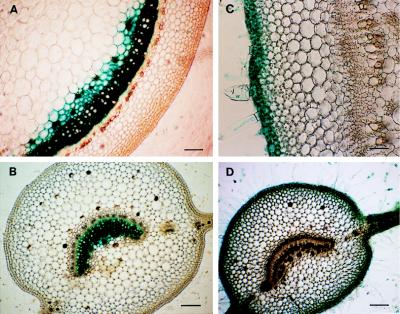
To further investigate the regulation of the tissue-specific expression of Pt4CL1 and Pt4CL2 genes at the cellular level, their promoter activities were analyzed in transgenic tobacco plants by histochemical staining of GUS gene expression driven by a 1-kb Pt4CL1 and 1.2-kb Pt4CL2 promoter sequences, respectively. In Pt4CL1p-GUS transgenic plants, intense GUS staining was detected in lignifying xylem of stem (Fig. 4A). Strong GUS activity also was found localized to xylem of leaf mid-rib (Fig. 4B) and of root (data not shown). However, there was no GUS expression in leaf blade, stem epidermis, cortex, phloem and pith, and flower petal. These results are consistent with the evidence that Pt4CL1 gene expression is xylem- or lignifying tissue-specific, and with the observation that Pt4CL1 mRNA level is the highest in aspen secondary developing xylem (Fig. 3B). In striking contrast to the Pt4CL1 promoter activity, the Pt4CL2 promoter did not direct GUS expression in vascular and xylem tissues in the stem and the leaf of Pt4CL2p-GUS transgenic plants. Instead, it directed GUS expression in lignin-deficient epidermal cells of the stem (Fig. 4C) and of the leaf (Fig. 4D), reflecting the association of Pt4CL2 with nonlignin-related phenylpropanoid biosynthesis in the plant’s outer layers. In addition, GUS staining also was detected in Pt4CL2p-GUS transgenic plant’s floral organs, such as stigma and petal (data not shown), suggesting the likely relevance of Pt4CL2 in mediating the formation of flavonoids, which are known to be accumulated in these organs (4, 28, 29).
Figure 4.
Histochemical analysis of GUS gene expression in transgenic tobacco. Transverse sections of the stem (A, bar = 100 μm) and leaf (B, bar = 100 μm) excised from Pt4CL1p-GUS transgenic tobacco, and transverse sections of the stem (C, bar = 250 μm) and leaf (D, bar = 100 μm) from Pt4CL2p-GUS transgenic tobacco were stained for GUS activity as described (17).
The epidermis-specific Pt4CL2 promoter activity indicated that the in vivo Pt4CL2 mRNA message observed in aspen stem internodes (Fig. 3B) could be caused by the signal derived from the epidermis RNA. However, this result could not be demonstrated in a Northern blot using a mixture of RNAs isolated from intact stem internodes. Therefore, we repeated the Northern blot analysis of RNAs isolated separately from epidermal layers and developing xylem of aspen stem internodes (one to 25 from shoot tip). Based on the Northern analysis, it was conclusive that the expression of Pt4CL2 mRNA is absent from developing xylem tissues but is evident in epidermal layers (Fig. 5), providing clarification that Pt4CL2 is epidermis-specific in aspen stem. Thus, the specific expression of Pt4CL2 mRNA in epidermis further supports the biochemical functions of Pt4CL2 protein in the biosynthesis of nonlignin-related phenylpropanoids. When the Northern blot was reprobed with Pt4CL1 cDNA, the results further validated that Pt4CL1 mRNA is specifically expressed in developing xylem but not in epidermal layers (Fig. 5), consistent with the lignin biosynthesis-associated catalytic activities of Pt4CL1.
Figure 5.
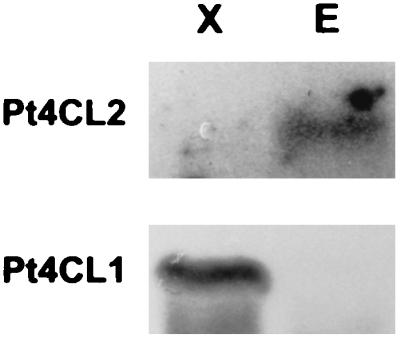
Northern blot analysis of total RNA (20 μg per lane) from epidermal layers (lane E) and developing xylem (X) of aspen stem (one to 25 internodes), probed with 32P-labeled Pt4CL1 and Pt4CL2 cDNAs, respectively.
A few studies (30, 31) demonstrated that the control of reporter GUS gene expression requires sequences downstream from those generally considered to comprise a promoter. Although this intragenic control of gene expression is very unusual in plants, the involvement of such control is indicated usually by the inconsistency between the gene promoter-driven GUS expression in transgenic plants and the in vivo expression of the genes of interest (31). However, the results of our promoter-GUS fusion analyses (Fig. 4) were in excellent agreement with the in vivo expression patterns of Pt4CL1 and Pt4CL2 genes in aspen (Fig. 3 and 5). Therefore, we believe that the promoter fragments incorporated in Pt4CL1p-GUS and Pt4CL2p-GUS fusion genes must encompass the regulatory sequence elements that are responsible for the contrasting tissue-specific expression between Pt4CL1 and Pt4CL2 genes in aspen. Thus, based on both in vivo gene expression and gene promoter activity analyses, we concluded that the expression of Pt4CL1 and Pt4CL2 genes in aspen is compartmentalized.
Our results demonstrate that in aspen two functionally distinct 4CLs are uniquely compartmentalized by their gene regulatory systems for mediating differentially the biosynthesis of lignin and other phenylpropanoids that serve different physiological functions in aspen. Pt4CL1 is involved in channeling hydroxycinnamic acid derivatives to the synthesis of guaiacyl-syringyl lignin in xylem tissues. Pt4CL2 is associated with the biosynthesis of phenylpropanoids other than lignin in epidermal cells in the stem and the leaf, suggesting its likely participation in disease-resistance or defense-related mechanisms in the plant’s outer layers. This study opens new avenues for investigating the distinct roles of 4CL isoforms in plant defense systems and in lignification in a tissue-specific manner. From a practical point of view, the tissue-specific Pt4CL1 and Pt4CL2 gene promoters may offer a more defined control of future genetic engineering of traits in trees that must be confined to xylem or epidermal cells.
Acknowledgments
We thank C. P. Joshi, S. A. Harding, and J. L. Popko for valuable comments on the manuscript. This research was supported by grants from U. S. Department of Agriculture National Research Initiative Competitive Grants Program (95–37103-2061), U. S. Department of Agriculture McIntire–Stennis Forestry Research Program, and Nippon Paper Industries Co.
ABBREVIATIONS
- 4CL
4-coumarate:CoA ligase
- Pt4CL
Populus tremuloides 4CL
- kb
kilobase
- GUS
β-glucouronidase
Footnotes
References
- 1.Mansell R L, Stöckigt J, Zenk M H. Z Pflanzenphysiol. 1972;68:286–288. [Google Scholar]
- 2.Knobloch K-H, Hahlbrock K. Eur J Biochem. 1975;52:311–320. doi: 10.1111/j.1432-1033.1975.tb03999.x. [DOI] [PubMed] [Google Scholar]
- 3.Hahlbrock K, Grisebach H. Annu Rev Plant Physiol. 1979;30:105–130. [Google Scholar]
- 4.Higuchi T. Biochemistry and Molecular Biology of Wood. New York: Springer; 1997. pp. 131–233. [Google Scholar]
- 5.Lee D, Douglas C J. Plant Physiol. 1996;112:193–205. doi: 10.1104/pp.112.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X-H, Chiang V L. Plant Physiol. 1997;113:65–74. doi: 10.1104/pp.113.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uhlmann A, Ebel J. Plant Physiol. 1993;102:1147–1156. doi: 10.1104/pp.102.4.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bugos R C, Chiang V L, Zhang X-H, Campbell E R, Podila G K, Campbell W H. BioTechniques. 1995;19:734–737. [PubMed] [Google Scholar]
- 9.Ge L, Chiang V L. Plant Physiol. 1996;112:861. [Google Scholar]
- 10.Li L, Popko J L, Zhang X-H, Osakabe K, Tsai C J, Joshi C P, Chiang V L. Proc Natl Acad Sci USA. 1997;94:5461–5466. doi: 10.1073/pnas.94.10.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsai, C. J., Popko, J. L., Mielke, M. R., Hu, W. J., Podila, G. K. & Chiang, V. L. (1998) Plant Physiol., in press. [DOI] [PMC free article] [PubMed]
- 12.Ranjeva R, Boudet A M, Faggion R. Biochimie (Paris) 1976;58:1255–1262. doi: 10.1016/s0300-9084(76)80125-3. [DOI] [PubMed] [Google Scholar]
- 13.Bradford M. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 14.Koncz C, Schell J. Mol Gen Genet. 1986;204:383–396. [Google Scholar]
- 15.Holsters M E, De Waele D, Depicker A, Messens E, Van Montagu M, Schell J. Mol Gen Genet. 1978;163:181–187. doi: 10.1007/BF00267408. [DOI] [PubMed] [Google Scholar]
- 16.Horsch R B, Fraley R T, Rogers S G, Klee H J, Hinchee M, Shah D M. Iowa State J Res. 1985;62:487–502. [Google Scholar]
- 17.Tsai C J, Podila G K, Chiang V L. Plant Cell Rep. 1994;14:94–97. doi: 10.1007/BF00233768. [DOI] [PubMed] [Google Scholar]
- 18.Lozoya E, Hoffmann H, Douglas C, Schulz W, Scheel D, Hahlbrock K. Eur J Biochem. 1988;176:661–667. doi: 10.1111/j.1432-1033.1988.tb14328.x. [DOI] [PubMed] [Google Scholar]
- 19.Bairoch A. Prosite: A Dictionary of Sites and Patterns in Protein. Switzerland: University of Geneva; 1991. . Release 8.00. [Google Scholar]
- 20.Becker-André M, Schulze-Lefert P, Hahlbrock K. J Biol Chem. 1991;266:8551–8559. [PubMed] [Google Scholar]
- 21.Yazaki K, Ogawa A, Tabata M. Plant Cell Physiol. 1995;36:1313–1329. [PubMed] [Google Scholar]
- 22.Bugos R C, Chiang V L, Campbell W H. Plant Mol Biol. 1991;17:1203–1215. doi: 10.1007/BF00028736. [DOI] [PubMed] [Google Scholar]
- 23.Kutsuki H, Shimada M, Higuchi T. Phytochemistry. 1982;21:267–271. [Google Scholar]
- 24.Higuchi T. In: Biosynthesis and Biodegradation of Wood Components. Higuchi T, editor. New York: Academic; 1985. pp. 141–160. [Google Scholar]
- 25.Gross G G, Mansell R L, Zenk M H. Biochem Physiol Pflanz. 1975;168:41–51. [Google Scholar]
- 26.Hahlbrock K, Scheel D, Logemann E, Nurnberger T, Parniske M, Reinold S, Sacks W R, Schmelzer E. Proc Natl Acad Sci USA. 1995;92:4150–4157. doi: 10.1073/pnas.92.10.4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Logemann E, Parniske M, Hahlbrock K. Proc Natl Acad Sci USA. 1995;92:5905–5909. doi: 10.1073/pnas.92.13.5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caldwell M M, Robberecht R, Flint SD. Physiol Plant. 1983;58:445–450. [Google Scholar]
- 29.Shirley B W. Trends Plant Sci. 1996;1:377–382. [Google Scholar]
- 30.Callis J, Fromm M, Walbot V. Genes Dev. 1987;1:1183–1200. doi: 10.1101/gad.1.10.1183. [DOI] [PubMed] [Google Scholar]
- 31.Sieburth L E, Meyerowitz E M. Plant Cell. 1997;9:355–365. doi: 10.1105/tpc.9.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]