Abstract
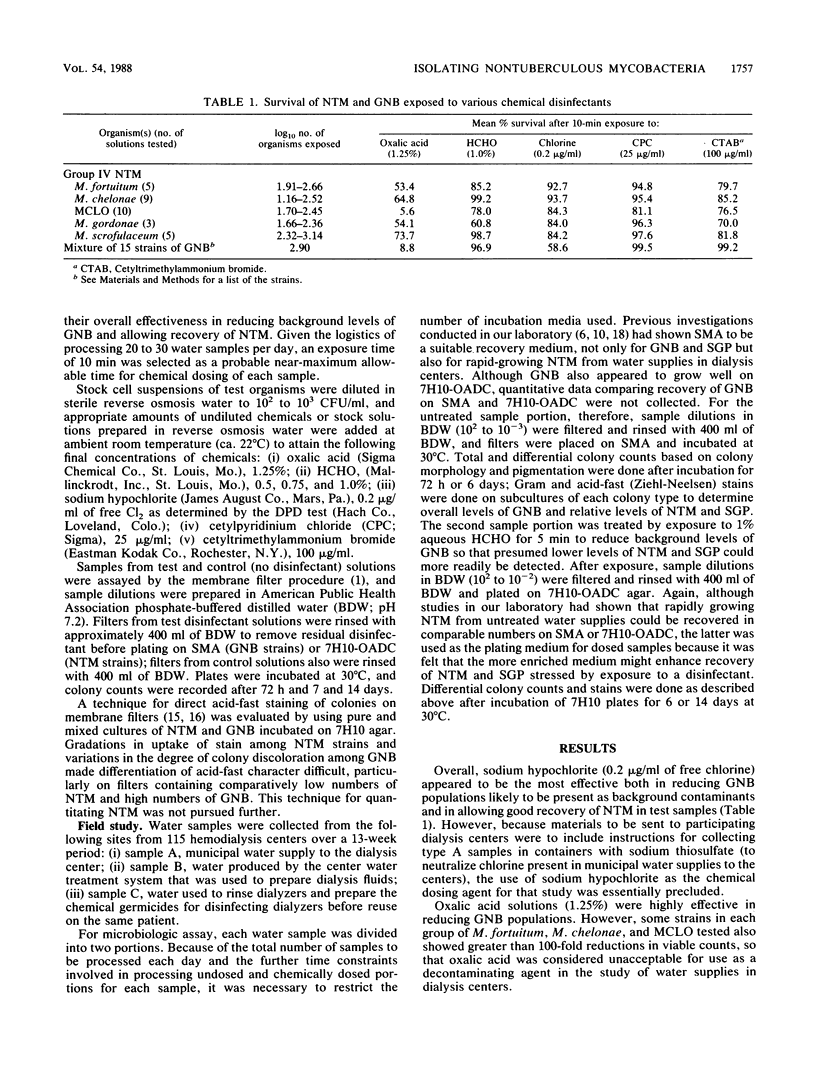
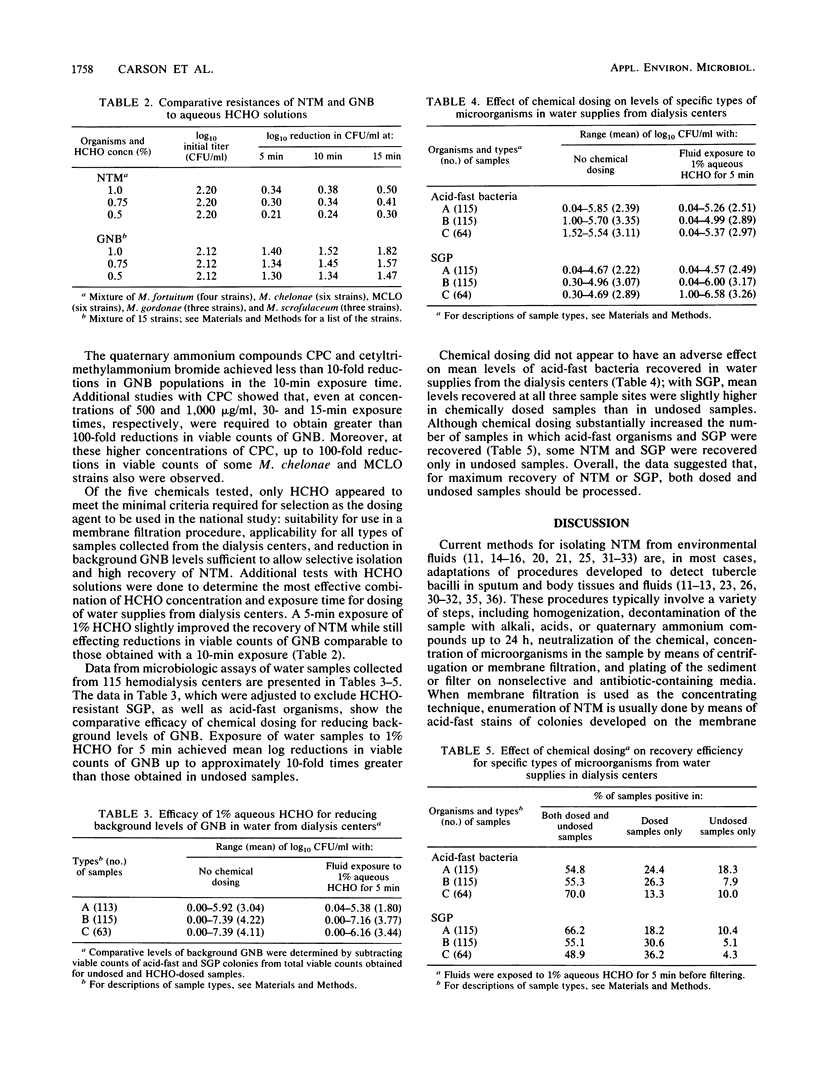
Investigations of nontuberculous mycobacterium (NTM) infections associated with various environmental sources have been hampered by the lack of adequate techniques for selective isolation of these organisms from environmental fluids. This study compared chemical dosing techniques for recovery of NTM from water samples collected from 115 randomly selected dialysis centers. Cell suspensions of NTM group II and IV isolates and gram-negative bacteria were exposed to solutions containing sodium hypochlorite (0.2 micrograms/ml of free available chlorine), formaldehyde (1, 0.75, or 0.5%), oxalic acid (1.25%), cetylpyridinium chloride (25 micrograms/ml), or cetyltrimethylammonium bromide (100 micrograms/ml). Results of standard membrane filtration assays with laboratory test strains and water samples from dialysis centers showed that 5 min of exposure to 1% formaldehyde effectively reduced gram-negative bacterial populations and allowed increased recovery of NTM in environmental fluids containing mixed microbial populations.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azadian B. S., Beck A., Curtis J. R., Cherrington L. E., Gower P. E., Phillips M., Eastwood J. B., Nicholls J. Disseminated infection with mycobacterium chelonei in a haemodialysis patient. Tubercle. 1981 Dec;62(4):281–284. doi: 10.1016/s0041-3879(81)80009-8. [DOI] [PubMed] [Google Scholar]
- Band J. D., Ward J. I., Fraser D. W., Peterson N. J., Silcox V. A., Good R. C., Ostroy P. R., Kennedy J. Peritonitis due to a mycobacterium chelonei-like organism associated with intermittent chronic peritoneal dialysis. J Infect Dis. 1982 Jan;145(1):9–17. doi: 10.1093/infdis/145.1.9. [DOI] [PubMed] [Google Scholar]
- Bland L., Alter M., Favero M., Carson L., Cusick L. Hemodialyzer reuse: practices in the United States and implication for infection control. Trans Am Soc Artif Intern Organs. 1985;31:556–559. [PubMed] [Google Scholar]
- Bolan G., Reingold A. L., Carson L. A., Silcox V. A., Woodley C. L., Hayes P. S., Hightower A. W., McFarland L., Brown J. W., 3rd, Petersen N. J. Infections with Mycobacterium chelonei in patients receiving dialysis and using processed hemodialyzers. J Infect Dis. 1985 Nov;152(5):1013–1019. doi: 10.1093/infdis/152.5.1013. [DOI] [PubMed] [Google Scholar]
- Borghans J. G., Stanford J. L. Mycobacterium chelonei in abscesses after injection of diphtheria-pertussis-tetanus-polio vaccine. Am Rev Respir Dis. 1973 Jan;107(1):1–8. doi: 10.1164/arrd.1973.107.1.1. [DOI] [PubMed] [Google Scholar]
- Brown T. H. The rapidly growing mycobacteria--Mycobacterium fortuitum and Mycobacterium chelonei. Infect Control. 1985 Jul;6(7):283–288. doi: 10.1017/s0195941700061762. [DOI] [PubMed] [Google Scholar]
- Carson L. A., Favero M. S., Bond W. W., Petersen N. J. Factors affecting comparative resistance of naturally occurring and subcultured Pseudomonas aeruginosa to disinfectants. Appl Microbiol. 1972 May;23(5):863–869. doi: 10.1128/am.23.5.863-869.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson L. A., Petersen N. J., Favero M. S., Aguero S. M. Growth characteristics of atypical mycobacteria in water and their comparative resistance to disinfectants. Appl Environ Microbiol. 1978 Dec;36(6):839–846. doi: 10.1128/aem.36.6.839-846.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins C. H., Grange J. M., Yates M. D. Mycobacteria in water. J Appl Bacteriol. 1984 Oct;57(2):193–211. doi: 10.1111/j.1365-2672.1984.tb01384.x. [DOI] [PubMed] [Google Scholar]
- Damato J. J., Collins M. T., Rothlauf M. V., McClatchy J. K. Detection of mycobacteria by radiometric and standard plate procedures. J Clin Microbiol. 1983 Jun;17(6):1066–1073. doi: 10.1128/jcm.17.6.1066-1073.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favero M. S., Petersen N. J., Carson L. A., Bond W. W., Hindman S. H. Gram-negative water bacteria in hemodialysis systems. Health Lab Sci. 1975 Oct;12(4):321–334. [PubMed] [Google Scholar]
- Gilardi G. L., Faur Y. C. Pseudomonas mesophilica and an unnamed taxon, clinical isolates of pink-pigmented oxidative bacteria. J Clin Microbiol. 1984 Oct;20(4):626–629. doi: 10.1128/jcm.20.4.626-629.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruft H., Falkinham J. O., 3rd, Parker B. C. Recent experience in the epidemiology of disease caused by atypical mycobacteria. Rev Infect Dis. 1981 Sep-Oct;3(5):990–996. doi: 10.1093/clinids/3.5.990. [DOI] [PubMed] [Google Scholar]
- Hayes P. S., McGiboney D. L., Band J. D., Feeley J. C. Resistance of Mycobacterium chelonei-like organisms to formaldehyde. Appl Environ Microbiol. 1982 Mar;43(3):722–724. doi: 10.1128/aem.43.3.722-724.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matajack M. L., Bissett M. L., Schifferle D., Wood R. M. Evaluation of a selective medium for mycobacteria. Am J Clin Pathol. 1973 Mar;59(3):391–397. doi: 10.1093/ajcp/59.3.391. [DOI] [PubMed] [Google Scholar]
- Powell B. L., Jr, Steadham J. E. Improved technique for isolation of Mycobacterium kansasii from water. J Clin Microbiol. 1981 May;13(5):969–975. doi: 10.1128/jcm.13.5.969-975.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothlauf M. V., Brown G. L., Blair E. B. Isolation of mycobacteria from undecontaminated specimens with selective 7H10 medium. J Clin Microbiol. 1981 Jan;13(1):76–79. doi: 10.1128/jcm.13.1.76-79.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell A. D., Hammond S. A., Morgan J. R. Bacterial resistance to antiseptics and disinfectants. J Hosp Infect. 1986 May;7(3):213–225. doi: 10.1016/0195-6701(86)90071-x. [DOI] [PubMed] [Google Scholar]
- Smith S. M., Eng R. H., Forrester C. Pseudomonas mesophilica infections in humans. J Clin Microbiol. 1985 Mar;21(3):314–317. doi: 10.1128/jcm.21.3.314-317.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithwick R. W., Stratigos C. B. Acid-fast microscopy on polycarbonate membrane filter sputum sediments. J Clin Microbiol. 1981 Jun;13(6):1109–1113. doi: 10.1128/jcm.13.6.1109-1113.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithwick R. W., Stratigos C. B., David H. L. Use of cetylpyridinium chloride and sodium chloride for the decontamination of sputum specimens that are transported to the laboratory for the isolation of Mycobacterium tuberculosis. J Clin Microbiol. 1975 May;1(5):411–413. doi: 10.1128/jcm.1.5.411-413.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songer J. G. Methods for selective isolation of mycobacteria from the environment. Can J Microbiol. 1981 Jan;27(1):1–7. doi: 10.1139/m81-001. [DOI] [PubMed] [Google Scholar]
- Steadham J. E. High-catalase strains of Mycobacterium kansasii isolated from water in Texas. J Clin Microbiol. 1980 May;11(5):496–498. doi: 10.1128/jcm.11.5.496-498.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson J. M., Thornsberry C., Silcox V. A. Rapidly growing mycobacteria: testing of susceptibility to 34 antimicrobial agents by broth microdilution. Antimicrob Agents Chemother. 1982 Aug;22(2):186–192. doi: 10.1128/aac.22.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazir M., David H. L., Boulahbal F. Evaluation of the chloride and bromide salts of cetylpyridium for the transportation of sputum in tuberculosis bacteriology. Tubercle. 1979 Mar;60(1):31–36. doi: 10.1016/0041-3879(79)90053-9. [DOI] [PubMed] [Google Scholar]
- Thoen C. O., Richards W. D., Jarnagin J. L. Comparison of six methods for isolating mycobacteria from swine lymph nodes. Appl Microbiol. 1974 Mar;27(3):448–451. doi: 10.1128/am.27.3.448-451.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Moulin G. C., Stottmeier K. D. Use of cetylpyridinium chloride in the decontamination of water for culture of mycobacteria. Appl Environ Microbiol. 1978 Nov;36(5):771–773. doi: 10.1128/aem.36.5.771-773.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


