Abstract
Nitrile hydratase, which occurs abundantly in cells of Rhodococcus rhodochrous J1 isolated from soil samples, catalyzes the hydration of 3-cyanopyridine to nicotinamide. By using resting cells, the reaction conditions for nicotinamide production were optimized. Under the optimum conditions, 100% of the added 12 M 3-cyanopyridine was converted to nicotinamide without the formation of nicotinic acid, and the highest yield achieved was 1,465 g of nicotinamide per liter of reaction mixture containing resting cells (1.48 g as dry cell weight) in 9 h. The nicotinamide produced was crystallized and then identified physicochemically. The further conversion of the nicotinamide to nicotinic acid was due to the low activity of nicotinamide as a substrate for the amidase(s) present in this organism.
Full text
PDF

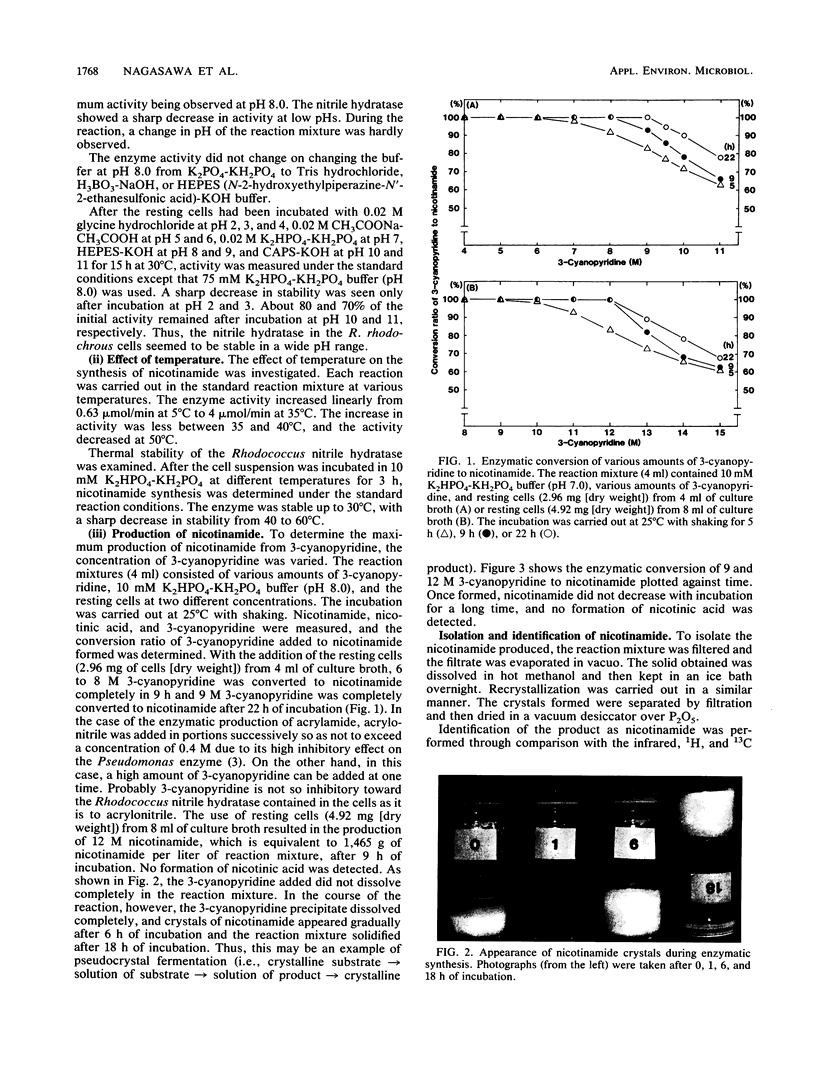
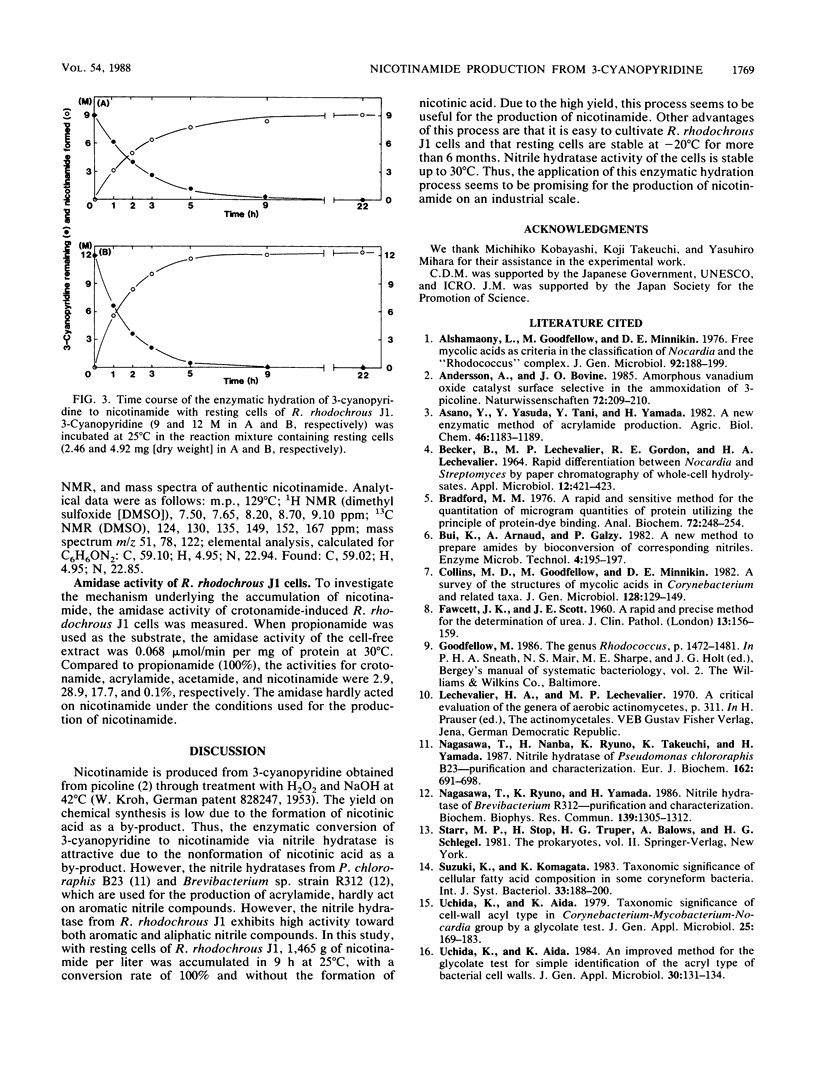
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alashamaony L., Goodfellow M., Minnikin D. E. Free mycolic acids as criteria in the classification of Nocardia and the 'rhodochrous' complex. J Gen Microbiol. 1976 Jan;92(1):188–199. doi: 10.1099/00221287-92-1-188. [DOI] [PubMed] [Google Scholar]
- BECKER B., LECHEVALIER M. P., GORDON R. E., LECHEVALIER H. A. RAPID DIFFERENTIATION BETWEEN NOCARDIA AND STREPTOMYCES BY PAPER CHROMATOGRAPHY OF WHOLE-CELL HYDROLYSATES. Appl Microbiol. 1964 Sep;12:421–423. doi: 10.1128/am.12.5.421-423.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Collins M. D., Goodfellow M., Minnikin D. E. A survey of the structures of mycolic acids in Corynebacterium and related taxa. J Gen Microbiol. 1982 Jan;128(1):129–149. doi: 10.1099/00221287-128-1-129. [DOI] [PubMed] [Google Scholar]
- FAWCETT J. K., SCOTT J. E. A rapid and precise method for the determination of urea. J Clin Pathol. 1960 Mar;13:156–159. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T., Nanba H., Ryuno K., Takeuchi K., Yamada H. Nitrile hydratase of Pseudomonas chlororaphis B23. Purification and characterization. Eur J Biochem. 1987 Feb 2;162(3):691–698. doi: 10.1111/j.1432-1033.1987.tb10692.x. [DOI] [PubMed] [Google Scholar]
- Nagasawa T., Ryuno K., Yamada H. Nitrile hydratase of Brevibacterium R312--purification and characterization. Biochem Biophys Res Commun. 1986 Sep 30;139(3):1305–1312. doi: 10.1016/s0006-291x(86)80320-5. [DOI] [PubMed] [Google Scholar]



