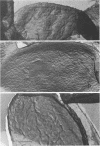
Abstract
The attachment of the conidia of the insect-pathogenic fungi Nomuraea rileyi, Beauveria bassiana, and Metarrhizium anisopliae to insect cuticle was mediated by strong binding forces. The attachment was passive and nonspecific in that the conidia adhered readily to both host and nonhost cuticle preparations. The hydrophobicity of the conidial wall and the insect epicuticle appeared to mediate the adhesion process. Detergents, solvents, and high-molecular-weight proteins known to neutralize hydrophobicity reduced conidial binding when added to conidium-cuticle preparations. However, these chemicals did not remove the hydrophobic components from the epicuticle or from conidial preparations. The outer surface of the conidium consists of a resilient layer of well-organized fascicles of rodlets. Intact rodlets extracted from B. bassiana conidia bound to insect cuticle and exhibited the hydrophobicity expressed by intact conidia. Both electrostatic charges and various hemagglutinin activities were also present on the conidial surface. However, competitive-inhibition studies indicated that these forces played little, if any, role in the adhesion process.
Full text
PDF

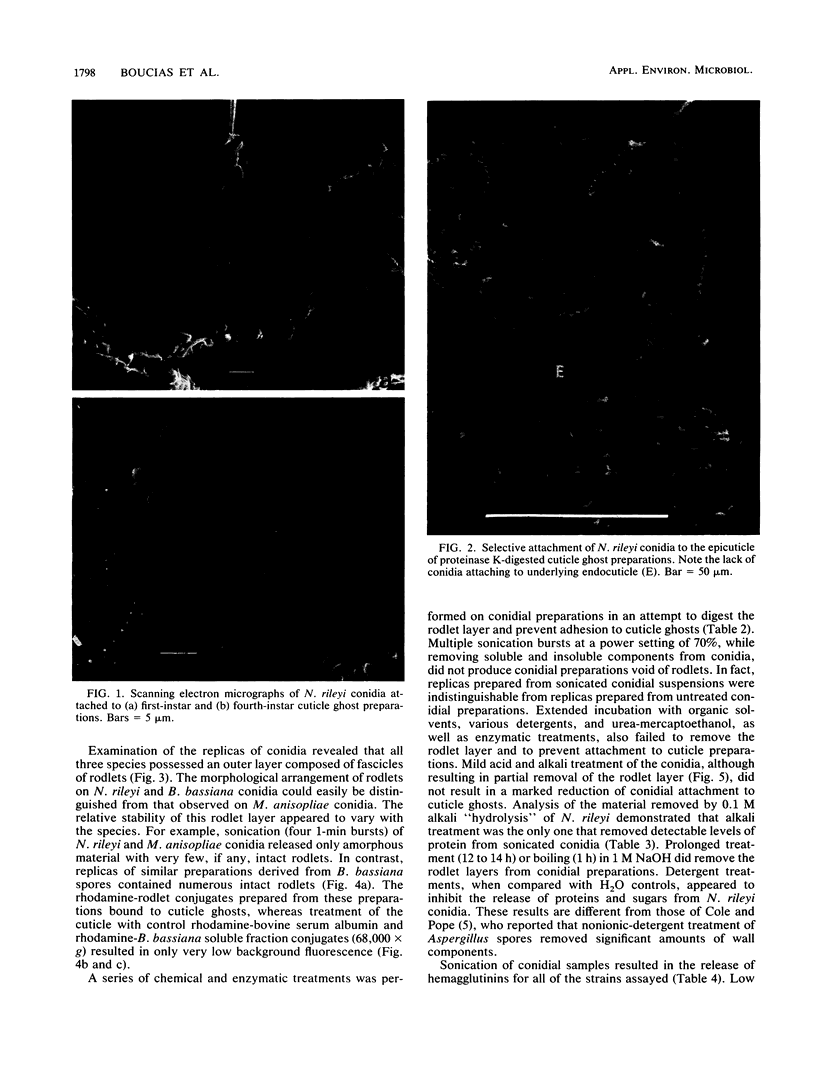
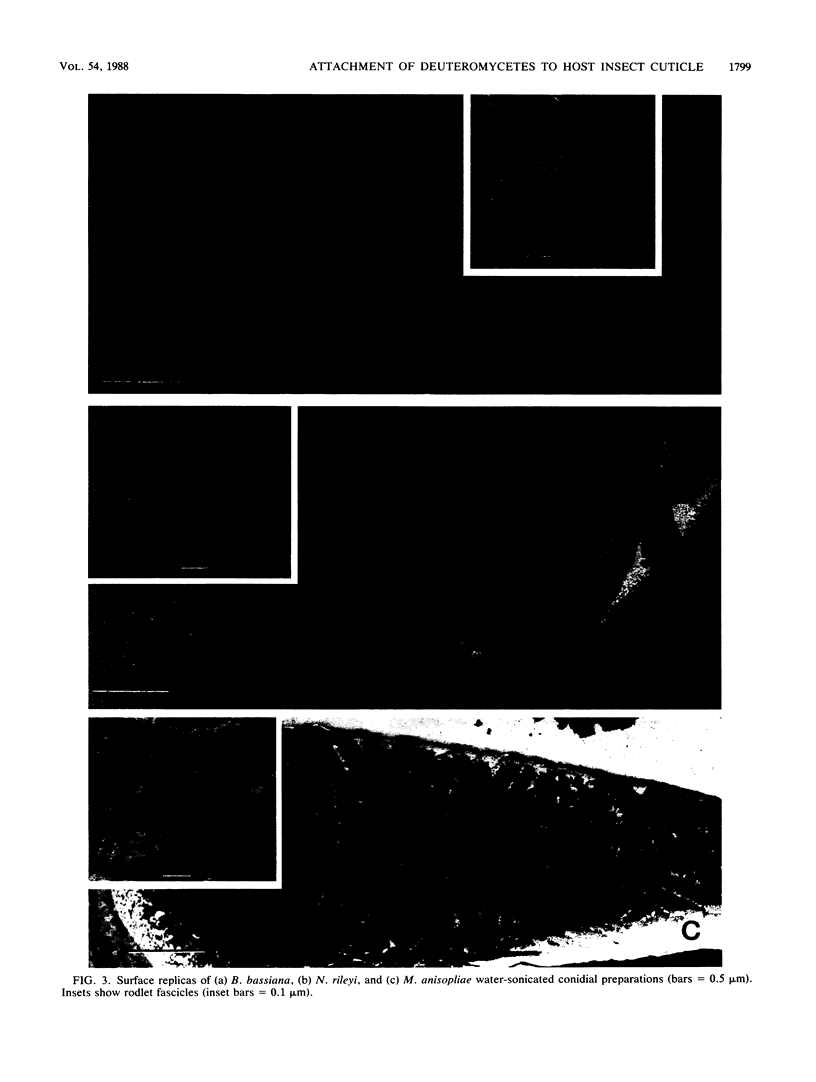
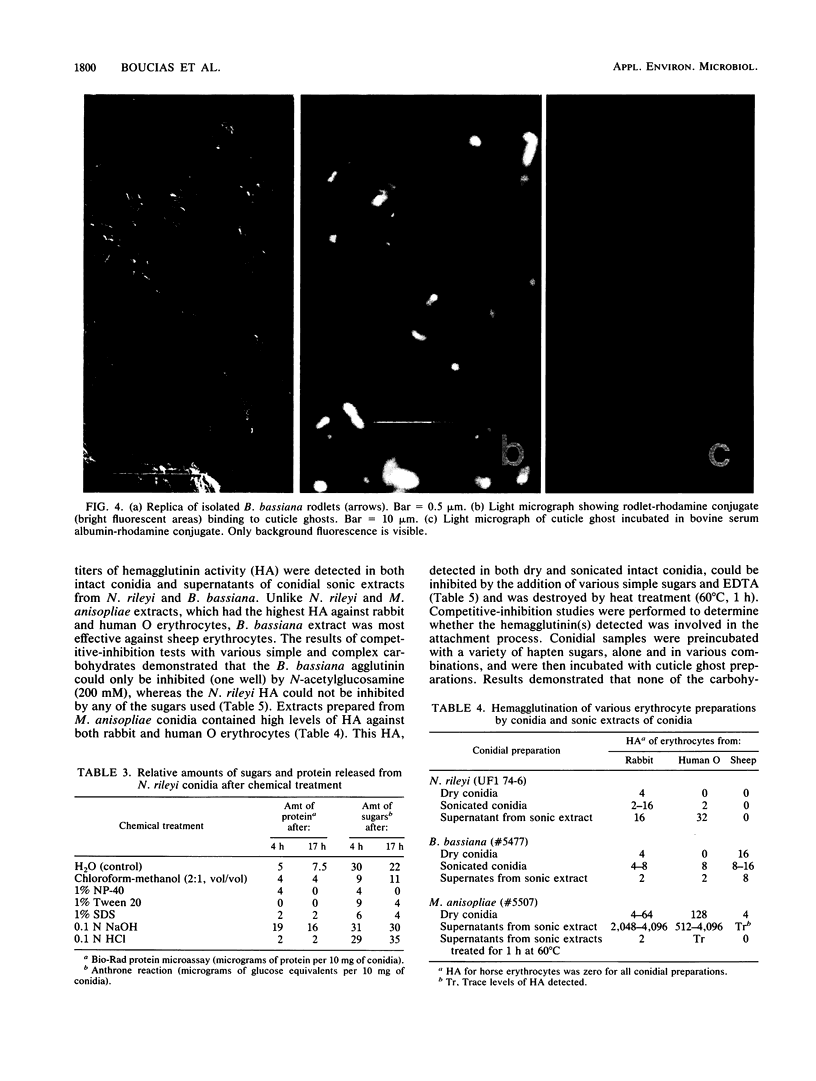

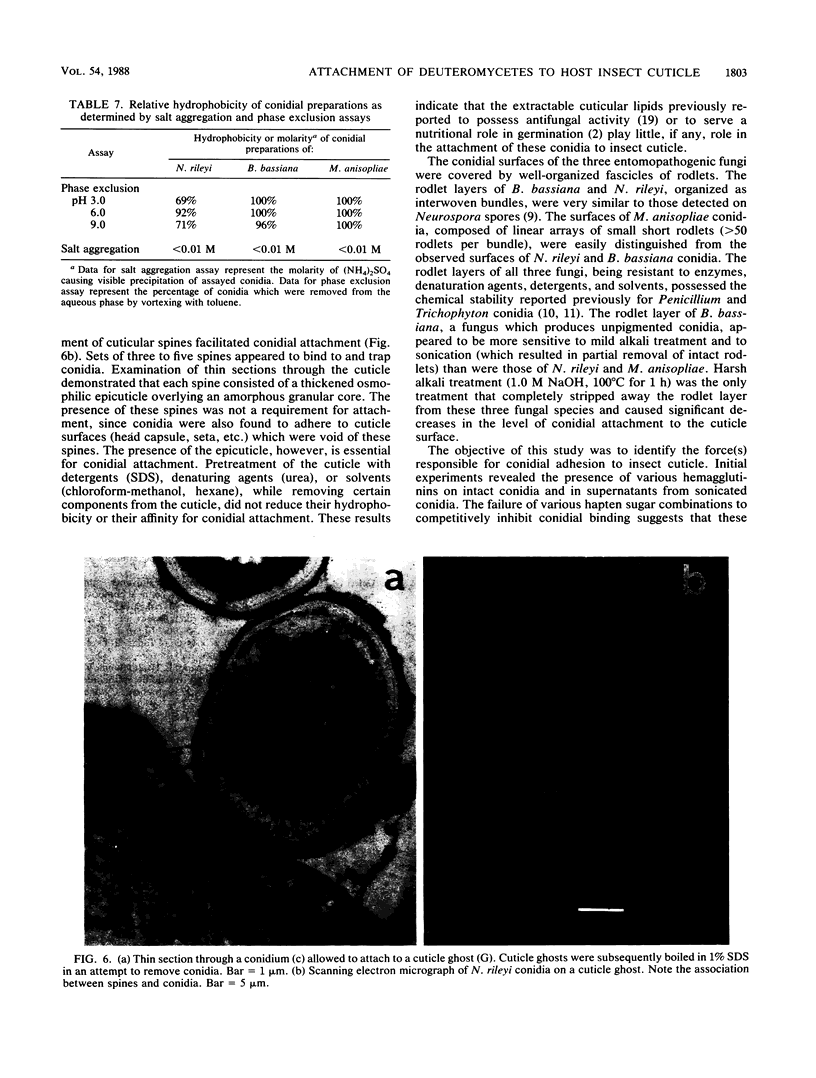

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beever R. E., Dempsey G. P. Function of rodlets on the surface of fungal spores. Nature. 1978 Apr 13;272(5654):608–610. doi: 10.1038/272608a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Gorelick F. S., Sarras M. P., Jr, Jamieson J. D. Regional differences in lectin binding to colonic epithelium by fluorescent and electron microscopy. J Histochem Cytochem. 1982 Nov;30(11):1097–1108. doi: 10.1177/30.11.6897257. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Wu-Yuan C. D., Blumenthal H. J. Isolation and characterization of the rodlet layer of Trichophyton mentagrophytes microconidial wall. J Bacteriol. 1976 Sep;127(3):1543–1549. doi: 10.1128/jb.127.3.1543-1549.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess W. M., Sassen M. M., Remsen C. C. Surface characteristics of Penicillum conidia. Mycologia. 1968 Mar-Apr;60(2):290–303. [PubMed] [Google Scholar]
- Pendland J. C., Boucias D. G. Hemagglutinin activity in the hemolymph of Anticarsia gemmatalis larvae infected with the fungus Nomuraea rileyi. Dev Comp Immunol. 1985 Winter;9(1):21–30. doi: 10.1016/0145-305x(85)90056-4. [DOI] [PubMed] [Google Scholar]
- Sassen M. M., Remsen C. C., Hess W. M. Fine structure of Penicillium megasporum conidiospores. Protoplasma. 1967;64(1):75–88. doi: 10.1007/BF01257383. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]