Abstract
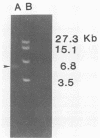
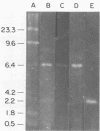
Aseptic isolation of the facultative gut microflora of the tobacco hornworm, Manduca sexta, yielded four microorganisms. Two were gram-positive Bacillus spp., one was Serratia plymuthica, and another was the yeast Candida guilliermondii. The three bacterial species were screened for extrachromosomal DNA, and S. plymuthica was found to have a 6.4-kilobase plasmid, which was designated pCP-1.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balganesh M., Setlow J. K. Effect of chromosome homology of plasmid transformation and plasmid conjugal transfer in Haemophilus influenzae. Basic Life Sci. 1985;30:571–584. doi: 10.1007/978-1-4613-2447-8_40. [DOI] [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Smith D. I. Plasmid-determined resistance to antimicrobial agents. Annu Rev Microbiol. 1978;32:469–518. doi: 10.1146/annurev.mi.32.100178.002345. [DOI] [PubMed] [Google Scholar]
- Foster T. J. Plasmid-determined resistance to antimicrobial drugs and toxic metal ions in bacteria. Microbiol Rev. 1983 Sep;47(3):361–409. doi: 10.1128/mr.47.3.361-409.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimont P. A., Grimont F. The genus Serratia. Annu Rev Microbiol. 1978;32:221–248. doi: 10.1146/annurev.mi.32.100178.001253. [DOI] [PubMed] [Google Scholar]
- Le Minor L., Coynault C., Schwartz M. Déterminisme plasmidique du caractère "lactose positif" de Serratia liquefaciens. Ann Microbiol (Paris) 1974 Oct-Nov;125B(3):357–366. [PubMed] [Google Scholar]
- Lysenko O. Non-sporeforming bacteria pathogenic to insects: incidence and mechanisms. Annu Rev Microbiol. 1985;39:673–695. doi: 10.1146/annurev.mi.39.100185.003325. [DOI] [PubMed] [Google Scholar]
- Mahler I., Levinson H. S., Wang Y., Halvorson H. O. Cadmium- and mercury-resistant Bacillus strains from a salt marsh and from Boston Harbor. Appl Environ Microbiol. 1986 Dec;52(6):1293–1298. doi: 10.1128/aem.52.6.1293-1298.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhaus E. A. A Study of the Bacteria Associated with Thirty Species of Insects. J Bacteriol. 1941 Dec;42(6):757–790. doi: 10.1128/jb.42.6.757-790.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte W., Green L., Misra T. K., Silver S. Resistance to mercury and to cadmium in chromosomally resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1986 Apr;29(4):663–669. doi: 10.1128/aac.29.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]