Abstract
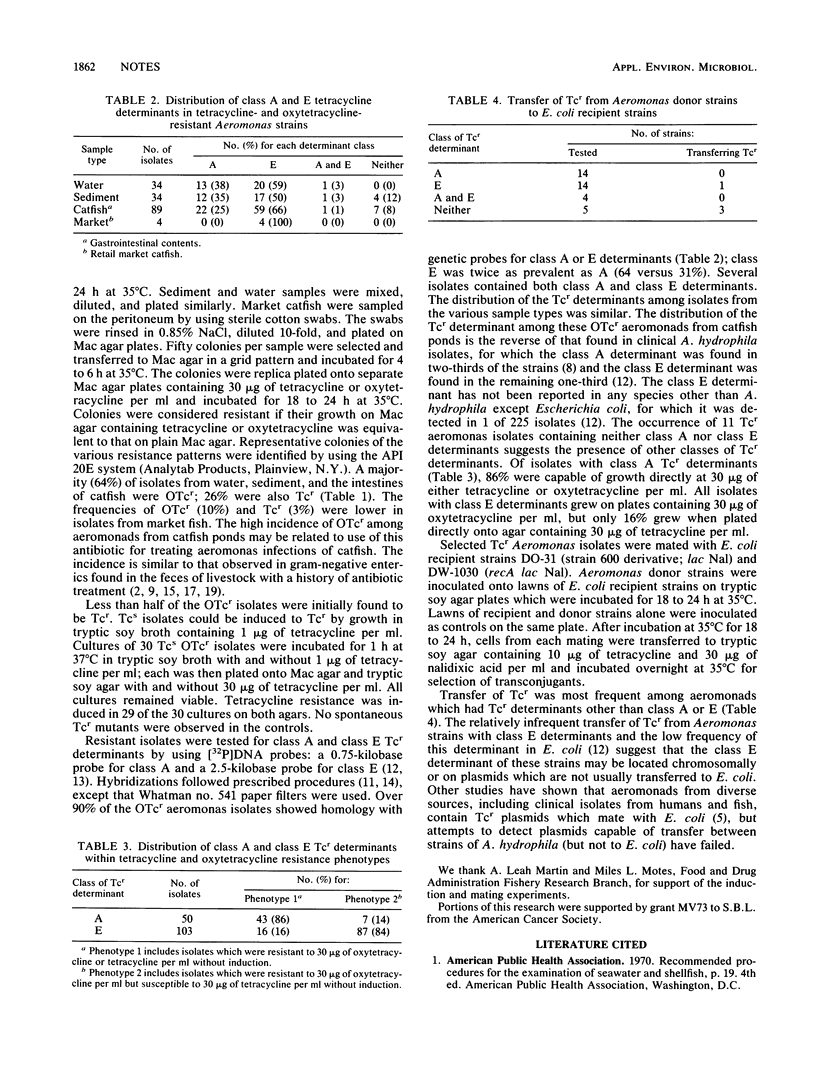
Oxytetracycline-resistant (OTcr) and tetracycline-resistant (Tcr) Aeromonas hydrophila were isolated commonly from catfish intestinal contents and the water and sediment of catfish culture ponds, but less frequently in market catfish. Isolates demonstrated two resistance phenotypes, Tcr OTcr and Tcs OTcr, when plated directly on to MacConkey agar containing 30 micrograms of tetracycline or oxytetracycline per ml. Tcs OTcr isolates expressed Tcr after induction by 1 h of growth in tryptic soy broth containing 1 microgram of tetracycline per ml. Over 90% of the resistant aeromonads hybridized with DNA probes for class A or class E Tcr determinants; class E was twice as prevalent as class A. The distribution of class A and E Tcr determinants varied with the Tcr phenotypes. Prior to induction, 86% of isolates with class A determinants were Tcr as well as OTcr, while only 16% with class E determinant were Tcr. Three of 5 Tcr aeromonads without class A or class E determinants and 1 of 14 with class E determinants transferred Tcr to Escherichia coli.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen M. L., Tauxe R. V. Drug-resistant Salmonella in the United States: an epidemiologic perspective. Science. 1986 Nov 21;234(4779):964–969. doi: 10.1126/science.3535069. [DOI] [PubMed] [Google Scholar]
- Holmberg S. D., Farmer J. J., 3rd Aeromonas hydrophila and Plesiomonas shigelloides as causes of intestinal infections. Rev Infect Dis. 1984 Sep-Oct;6(5):633–639. doi: 10.1093/clinids/6.5.633. [DOI] [PubMed] [Google Scholar]
- Holmberg S. D., Wells J. G., Cohen M. L. Animal-to-man transmission of antimicrobial-resistant Salmonella: investigations of U.S. outbreaks, 1971-1983. Science. 1984 Aug 24;225(4664):833–835. doi: 10.1126/science.6382605. [DOI] [PubMed] [Google Scholar]
- Levy S. B., FitzGerald G. B., Macone A. B. Changes in intestinal flora of farm personnel after introduction of a tetracycline-supplemented feed on a farm. N Engl J Med. 1976 Sep 9;295(11):583–588. doi: 10.1056/NEJM197609092951103. [DOI] [PubMed] [Google Scholar]
- Maas R. An improved colony hybridization method with significantly increased sensitivity for detection of single genes. Plasmid. 1983 Nov;10(3):296–298. doi: 10.1016/0147-619x(83)90045-8. [DOI] [PubMed] [Google Scholar]
- Marshall B., Morrissey S., Flynn P., Levy S. B. A new tetracycline-resistance determinant, class E, isolated from Enterobacteriaceae. Gene. 1986;50(1-3):111–117. doi: 10.1016/0378-1119(86)90315-x. [DOI] [PubMed] [Google Scholar]
- Marshall B., Tachibana C., Levy S. B. Frequency of tetracycline resistance determinant classes among lactose-fermenting coliforms. Antimicrob Agents Chemother. 1983 Dec;24(6):835–840. doi: 10.1128/aac.24.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P. The development and spread of antibiotic-resistant bacteria as a consequence of feeding antibiotics to livestock. Ann N Y Acad Sci. 1981;368:23–59. doi: 10.1111/j.1749-6632.1981.tb15430.x. [DOI] [PubMed] [Google Scholar]
- San Joaquin V. H., Scribner R. K., Pickett D. A., Welch D. F. Antimicrobial susceptibility of Aeromonas species isolated from patients with diarrhea. Antimicrob Agents Chemother. 1986 Nov;30(5):794–795. doi: 10.1128/aac.30.5.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel D., Huber W. G., Enloe F. Continuous non-therapeutic use of antibacterial drugs in feed and drug resistance of the gram-negative enteric florae of food-producing animals. Antimicrob Agents Chemother. 1974 Dec;6(6):697–701. doi: 10.1128/aac.6.6.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spika J. S., Waterman S. H., Hoo G. W., St Louis M. E., Pacer R. E., James S. M., Bissett M. L., Mayer L. W., Chiu J. Y., Hall B. Chloramphenicol-resistant Salmonella newport traced through hamburger to dairy farms. A major persisting source of human salmonellosis in California. N Engl J Med. 1987 Mar 5;316(10):565–570. doi: 10.1056/NEJM198703053161001. [DOI] [PubMed] [Google Scholar]


