Abstract
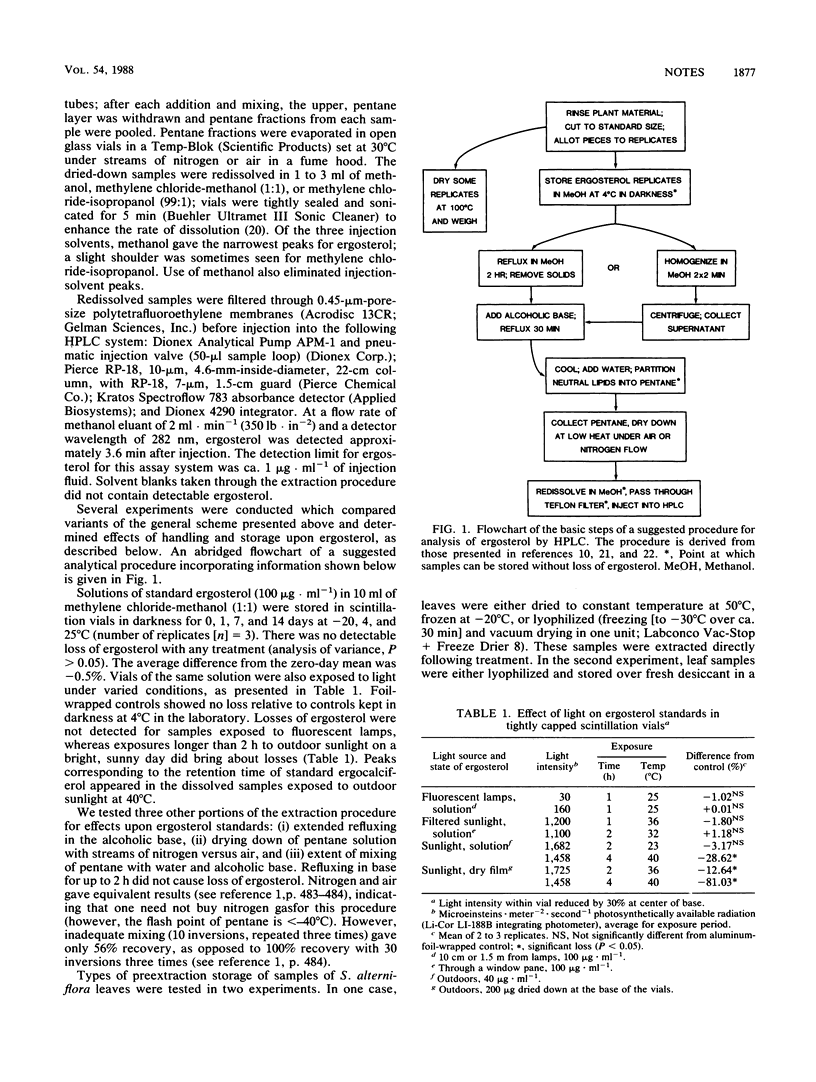
Portions of published procedures for measurement of ergosterol content of decomposing plants were examined for their influence upon ergosterol yield. Common methods of treatment of plant samples prior to sterol extraction (e.g., oven drying, freezing, lyophilization) led to reduced recoveries of ergosterol (ca. 20 to 80%). The least destructive method was direct placement and storage in methanol. Photoconversion of ergosterol is not likely to cause losses during analysis, but losses are likely if there is insufficient mixing during neutral-lipid partitioning from base-hydrolysis reagents. Homogenization (two times for 2 min) and refluxing (2 h) in methanol were equally effective in extracting ergosterol. Direct extraction in base-hydrolysis reagents was less effective (by ca. 40%).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gonzales R. A., Parks L. W. Acid-labilization of sterols for extraction from yeast. Biochim Biophys Acta. 1977 Dec 21;489(3):507–509. doi: 10.1016/0005-2760(77)90171-0. [DOI] [PubMed] [Google Scholar]
- Nes W. R., Dhanuka I. C., Pinto W. J. Evidence for facilitated transport in the absorption of sterols by Saccharomyces cerevisiae. Lipids. 1986 Jan;21(1):102–106. doi: 10.1007/BF02534311. [DOI] [PubMed] [Google Scholar]
- Rodriguez R. J., Parks L. W. High-performance liquid chromatography of sterols: yeast sterols. Methods Enzymol. 1985;111:37–51. doi: 10.1016/s0076-6879(85)11004-9. [DOI] [PubMed] [Google Scholar]
- Shapiro B. E., Gealt M. A. Ergosterol and lanosterol from Aspergillus nidulans. J Gen Microbiol. 1982 May;128(5):1053–1056. doi: 10.1099/00221287-128-5-1053. [DOI] [PubMed] [Google Scholar]
- Tsukida K. Analysis of vitamin D2 isomers. Methods Enzymol. 1980;67:326–335. doi: 10.1016/s0076-6879(80)67040-2. [DOI] [PubMed] [Google Scholar]


