Abstract
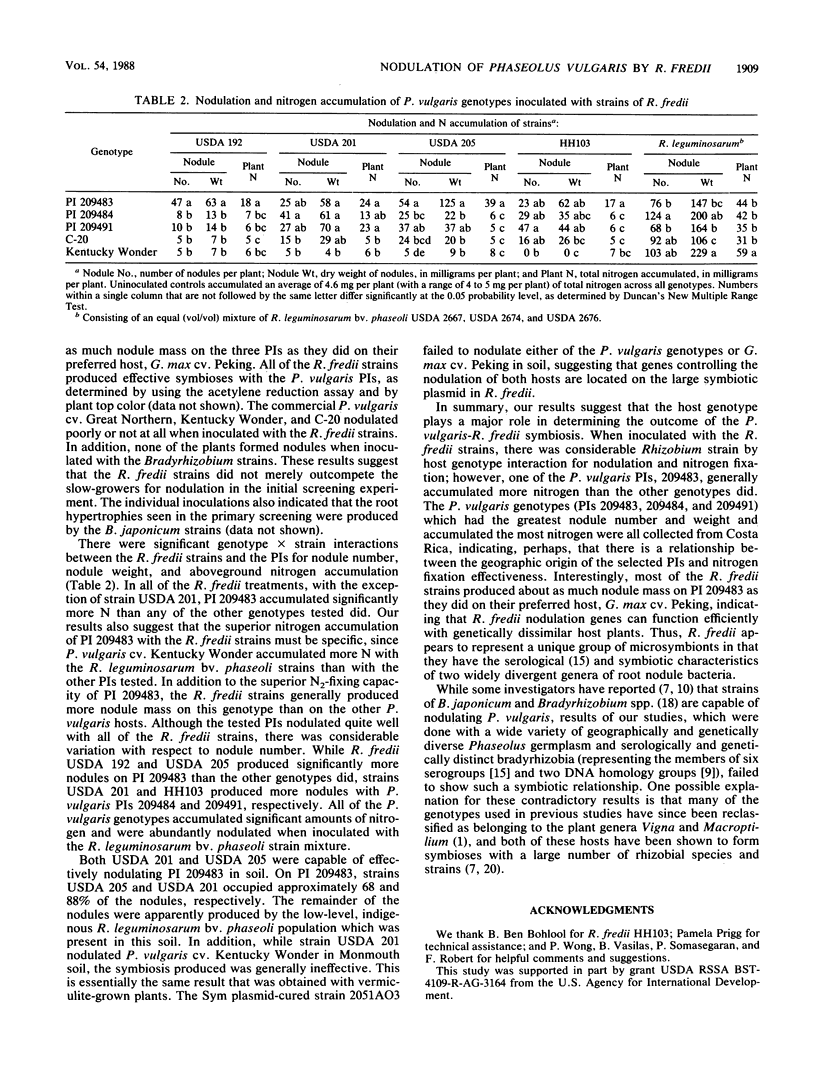
Phaseolus plant introduction (PI) genotypes (consisting of 684 P. vulgaris, 26 P. acutifolius, 39 P. lunatus, and 5 P. coccineus accessions) were evaluated for their ability to form effective symbioses with strains of six slow-growing (Bradyrhizobium) and four fast-growing (Rhizobium fredii) soybean rhizobia. Of the 684 P. vulgaris genotypes examined, three PIs were found to form effective nitrogen-fixing symbioses with the R. fredii strains. While none of the Bradyrhizobium strains nodulated any of the genotypes tested, some produced large numbers of undifferentiated root proliferations (hypertrophies). A symbiotic plasmid-cured R. fredii strain failed to nodulate the P. vulgaris PIs and cultivars, suggesting that P. vulgaris host range genes are Sym plasmid borne in the fast-growing soybean rhizobia.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barua M., Bhaduri P. N. Rhizobial relationship of the genus Phaseolus. I. Cross-inoculation performance of different species to R. phaseoli, R. japonicum, and the cowpea organism. Can J Microbiol. 1967 Jul;13(7):910–913. doi: 10.1139/m67-120. [DOI] [PubMed] [Google Scholar]
- Cregan P. B., Keyser H. H. Influence of Glycine spp. on Competitiveness of Bradyrhizobium japonicum and Rhizobium fredii. Appl Environ Microbiol. 1988 Mar;54(3):803–808. doi: 10.1128/aem.54.3.803-808.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eardly B. D., Hannaway D. B., Bottomley P. J. Characterization of Rhizobia from Ineffective Alfalfa Nodules: Ability to Nodulate Bean Plants [Phaseolus vulgaris (L.) Savi.]. Appl Environ Microbiol. 1985 Dec;50(6):1422–1427. doi: 10.1128/aem.50.6.1422-1427.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy R. W., Holsten R. D., Jackson E. K., Burns R. C. The acetylene-ethylene assay for n(2) fixation: laboratory and field evaluation. Plant Physiol. 1968 Aug;43(8):1185–1207. doi: 10.1104/pp.43.8.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowsky M. J., Bohlool B. B., Keyser H. H. Serological Relatedness of Rhizobium fredii to Other Rhizobia and to the Bradyrhizobia. Appl Environ Microbiol. 1987 Aug;53(8):1785–1789. doi: 10.1128/aem.53.8.1785-1789.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowsky M. J., Bohlool B. B. Possible involvement of a megaplasmid in nodulation of soybeans by fast-growing rhizobia from china. Appl Environ Microbiol. 1983 Oct;46(4):906–911. doi: 10.1128/aem.46.4.906-911.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E. L., Bakole R. O., Bohlool B. B. Fluorescent-antibody approach to study of rhizobia in soil. J Bacteriol. 1968 Jun;95(6):1987–1992. doi: 10.1128/jb.95.6.1987-1992.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shantharam S., Wong P. P. Recognition of leguminous hosts by a promiscuous Rhizobium strain. Appl Environ Microbiol. 1982 Mar;43(3):677–685. doi: 10.1128/aem.43.3.677-685.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


