Abstract
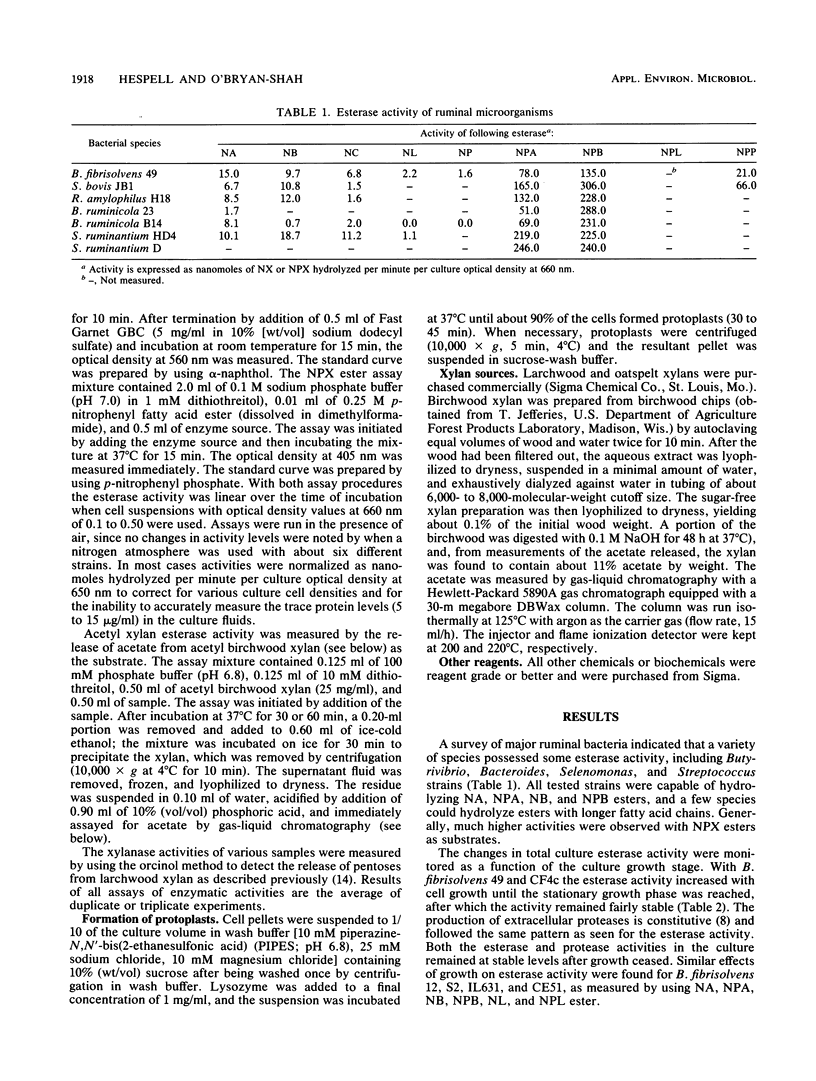
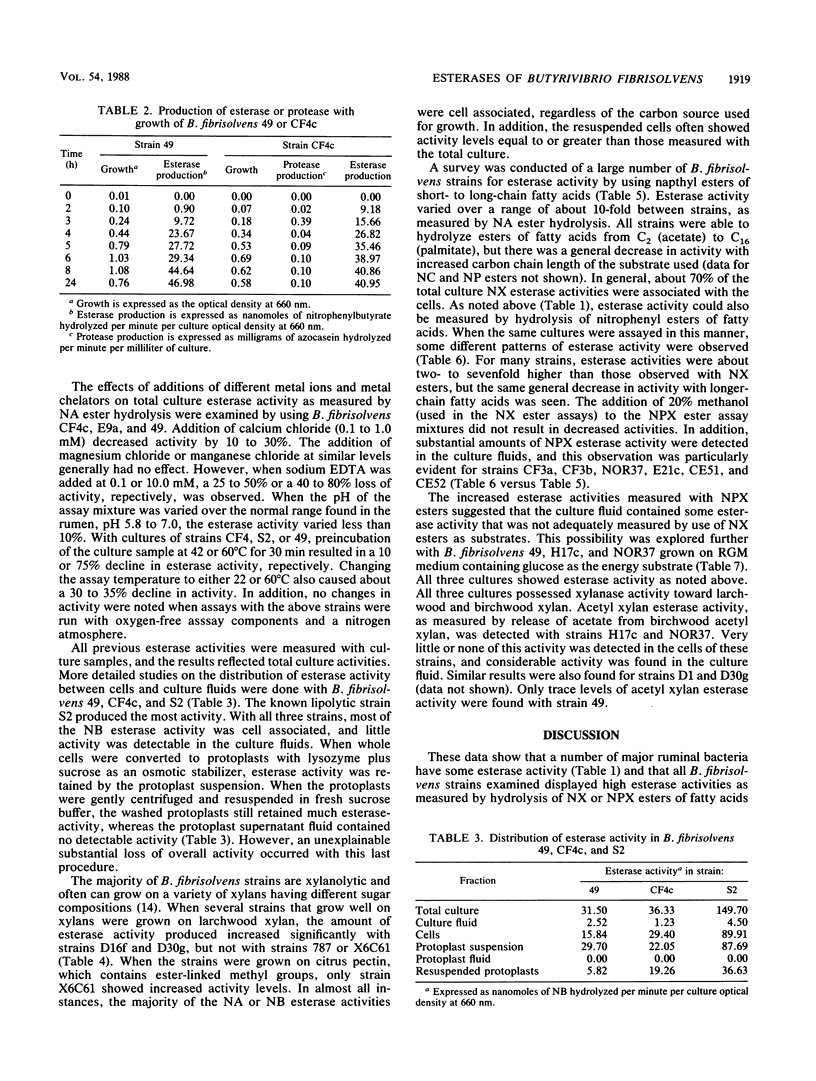
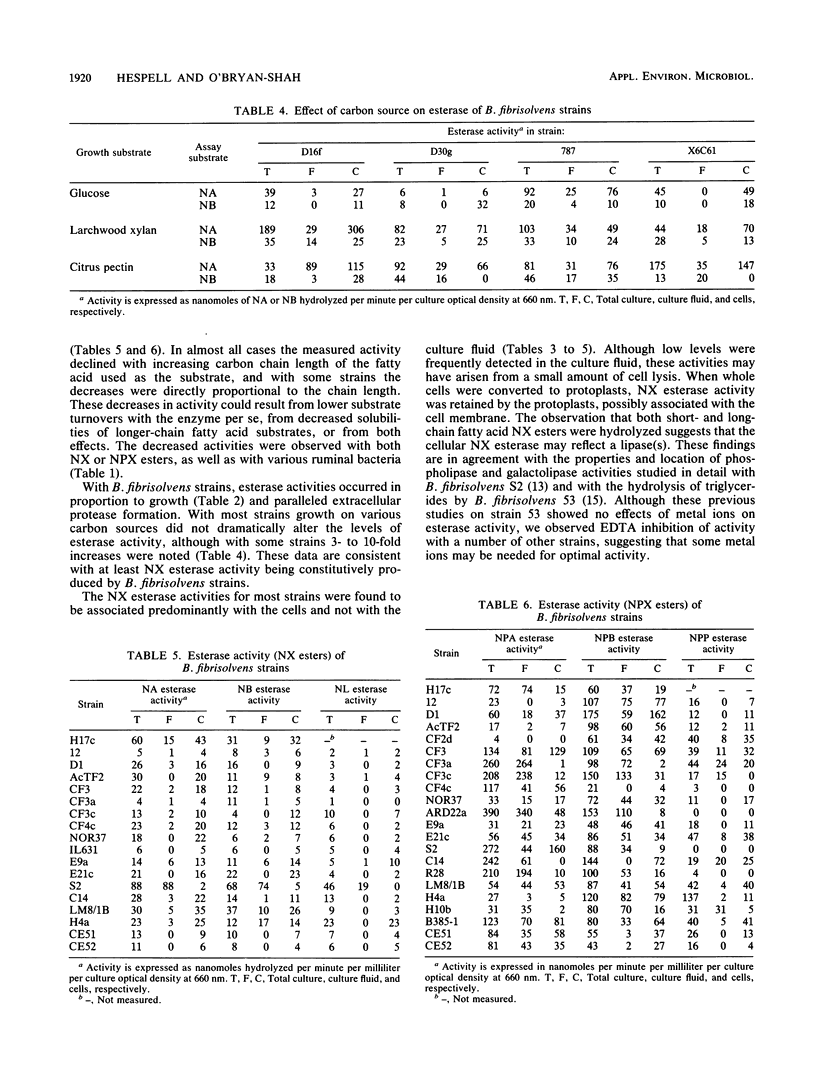
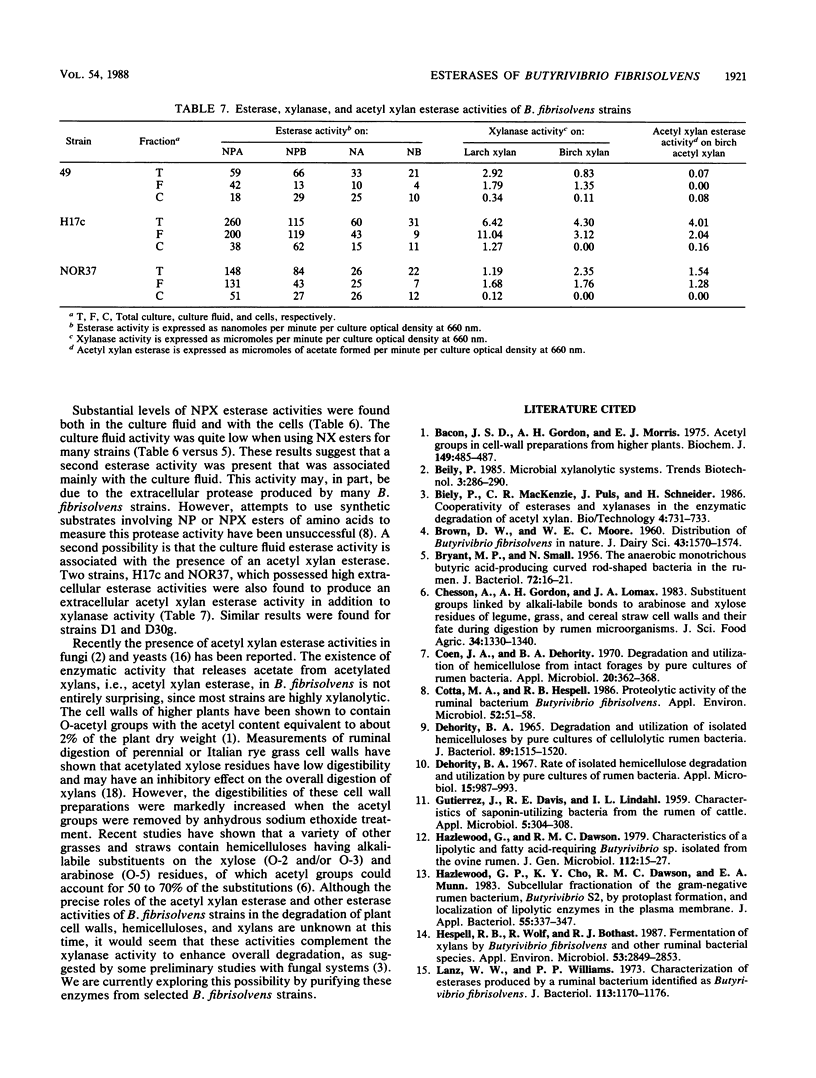
Thirty strains of Butyrivibrio fibrisolvens isolated in diverse geographical locations were examined for esterase activity by using naphthyl esters of acetate, butyrate, caprylate, laurate, and palmitate. All strains possessed some esterase activity, and high levels of activity were observed with strains 49, H17c, S2, AcTF2, and LM8/1B. Esterase activity also was detected in other ruminal bacteria (Bacteroides ruminicola, Selenomonas ruminantium, Ruminobacter amylophilus, and Streptococcus bovis). For all B. fibrisolvens strains tested, naphthyl fatty acid esterase activity paralleled culture growth and was predominantly cell associated. With strains 49, CF4c, and S2, the activity was retained by protoplasts made from whole cells. Esterase activity was detected with all strains when grown on glucose, and some strains showed higher activity levels when grown on other substrates (larchwood xylan or citrus pectin). When nitrophenyl esters of fatty acids were used to measure esterase activity, generally four- to sevenfold-higher activity levels were detected, and with a number of strains substantial levels were found in the culture fluid. Cultures of these strains (H17c, NOR37, D1, and D30g) contained xylanase and acetyl xylan esterase activities, neither of which was associated to any great extent with the cells. Acetyl xylan esterase has not been previously detected in ruminal bacteria and may be important to overall digestion of forage by these organisms.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRYANT M. P., SMALL N. The anaerobic monotrichous butyric acid-producing curved rod-shaped bacteria of the rumen. J Bacteriol. 1956 Jul;72(1):16–21. doi: 10.1128/jb.72.1.16-21.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon J. S., Gordon A. H., Morris E. J. Acetyl groups in cell-wall preparations from higher plants. Biochem J. 1975 Aug;149(2):485–487. doi: 10.1042/bj1490485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen J. A., Dehority B. A. Degradation and utilization of hemicellulose from intact forages by pure cultures of rumen bacteria. Appl Microbiol. 1970 Sep;20(3):362–368. doi: 10.1128/am.20.3.362-368.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotta M. A., Hespell R. B. Proteolytic activity of the ruminal bacterium Butyrivibrio fibrisolvens. Appl Environ Microbiol. 1986 Jul;52(1):51–58. doi: 10.1128/aem.52.1.51-58.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEHORITY B. A. DEGRADATION AND UTILIZATION OF ISOLATED HEMICELLULOSE BY PURE CULTURES OF CELLULOLYTIC RUMEN BACTERIA. J Bacteriol. 1965 Jun;89:1515–1520. doi: 10.1128/jb.89.6.1515-1520.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehority B. A. Rate of isolated hemicellulose degradation and utilization by pure cultures of rumen bacteria. Appl Microbiol. 1967 Sep;15(5):987–993. doi: 10.1128/am.15.5.987-993.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUTIERREZ J., DAVIS R. E., LINDAHL I. L. Characteristics of saponin-utilizing bacteria from the rumen of cattle. Appl Microbiol. 1959 Sep;7:304–308. doi: 10.1128/am.7.5.304-308.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlewood G., Dawson R. M. Characteristics of a lipolytic and fatty acid-requiring Butyrivibrio sp. isolated from the ovine rumen. J Gen Microbiol. 1979 May;112(1):15–27. doi: 10.1099/00221287-112-1-15. [DOI] [PubMed] [Google Scholar]
- Hespell R. B., Wolf R., Bothast R. J. Fermentation of xylans by Butyrivibrio fibrisolvens and other ruminal bacteria. Appl Environ Microbiol. 1987 Dec;53(12):2849–2853. doi: 10.1128/aem.53.12.2849-2853.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanz W. W., Williams P. P. Characterization of esterases produced by a ruminal bacterium identified as Butyrivibrio fibrisolvens. J Bacteriol. 1973 Mar;113(3):1170–1176. doi: 10.1128/jb.113.3.1170-1176.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., To R. J., Latta R. K., Biely P., Schneider H. Some Properties of Extracellular Acetylxylan Esterase Produced by the Yeast Rhodotorula mucilaginosa. Appl Environ Microbiol. 1987 Dec;53(12):2831–2834. doi: 10.1128/aem.53.12.2831-2834.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl Microbiol. 1974 May;27(5):961–979. doi: 10.1128/am.27.5.961-979.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack R. J. Neutral sugar composition of extracellular polysaccharides produced by strains of Butyrivibrio fibrisolvens. Appl Environ Microbiol. 1988 Apr;54(4):878–883. doi: 10.1128/aem.54.4.878-883.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlake K., Mackie R. I., Dutton M. F. T-2 toxin metabolism by ruminal bacteria and its effect on their growth. Appl Environ Microbiol. 1987 Mar;53(3):587–592. doi: 10.1128/aem.53.3.587-592.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


