Abstract
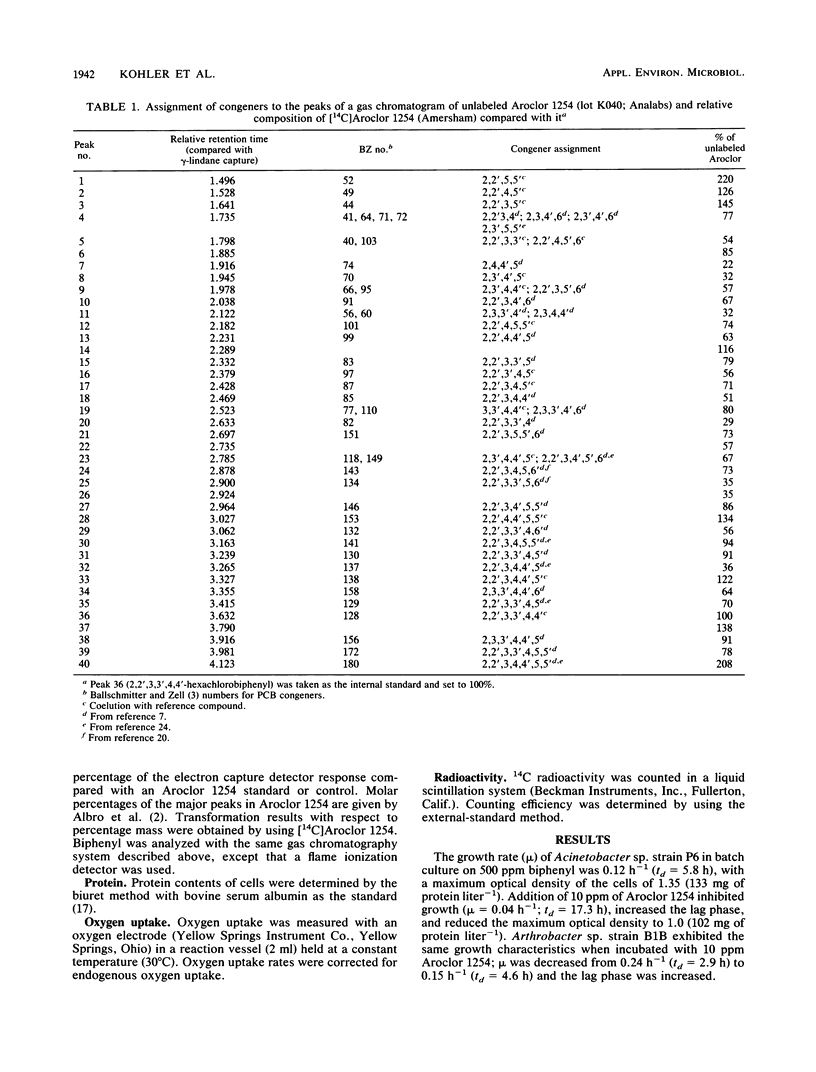
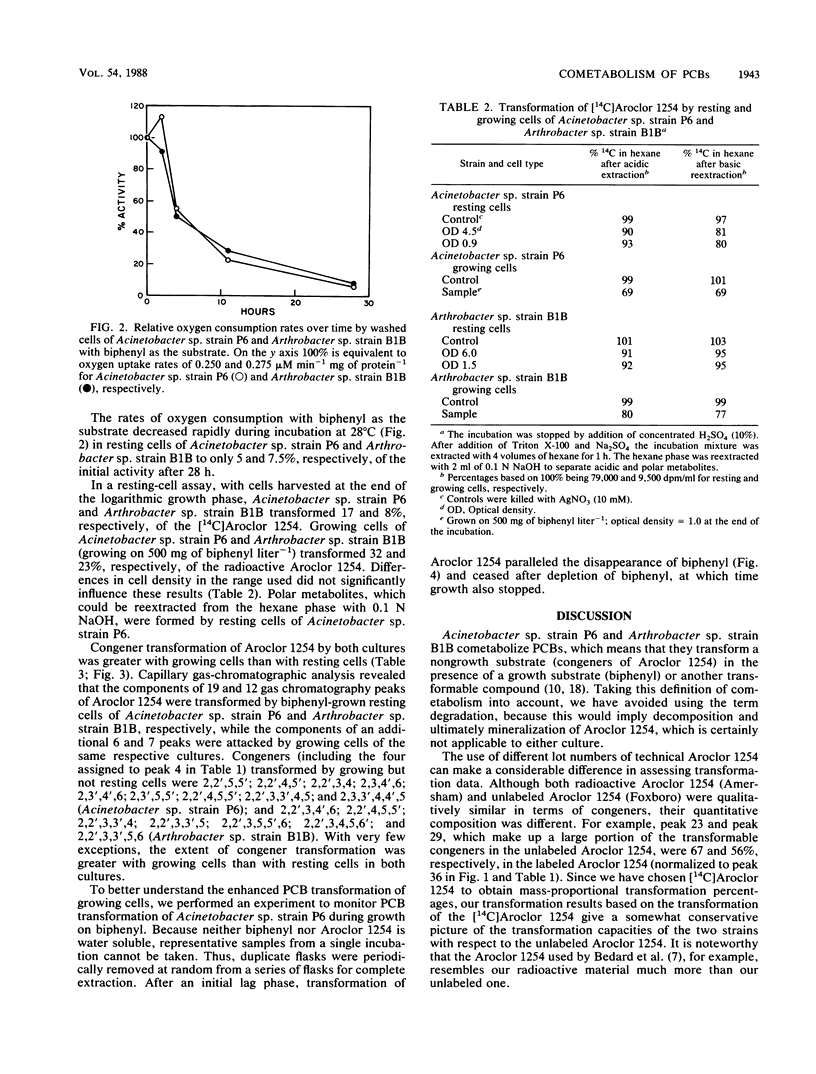
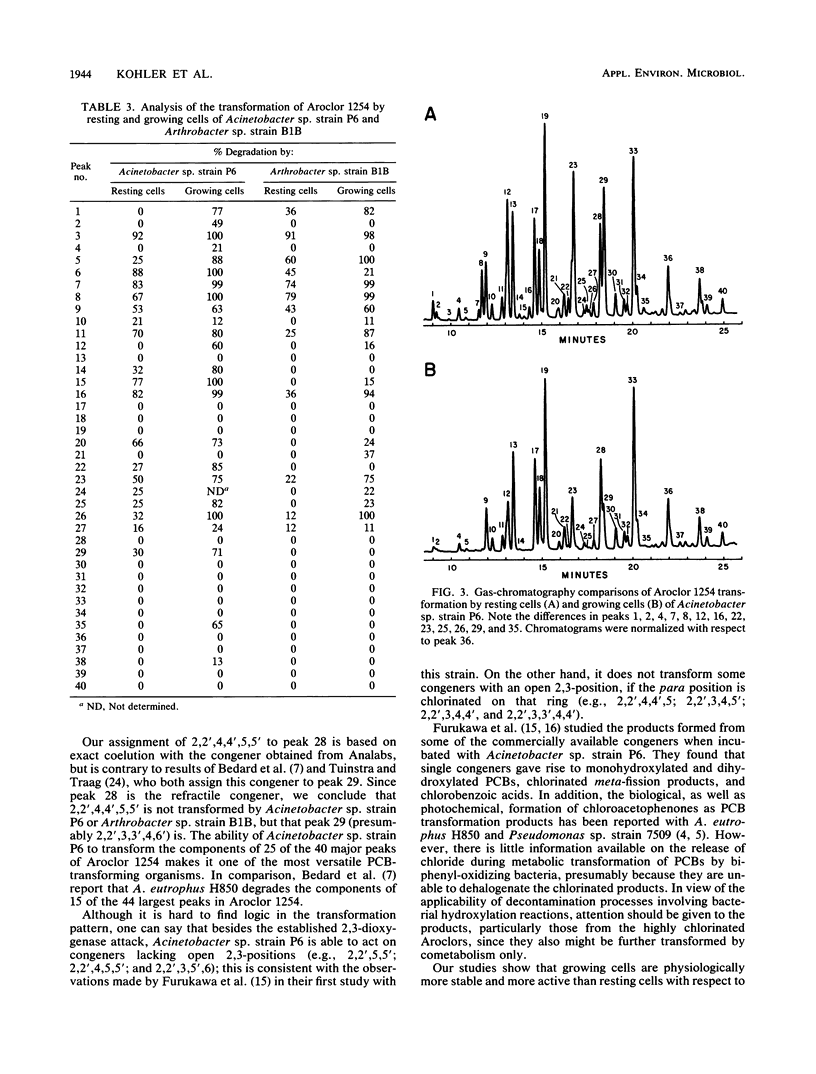
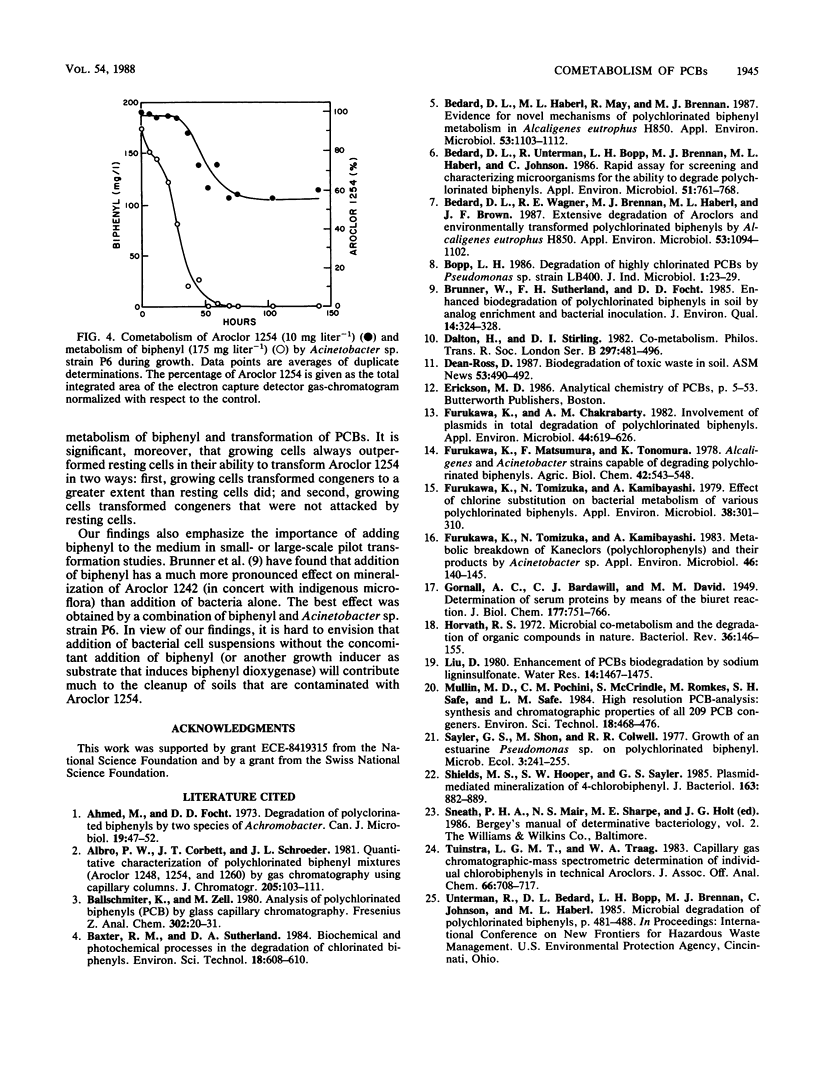
Acinetobacter sp. strain P6 and a soil isolate, Arthrobacter sp. strain B1B, were tested for their ability to transform Aroclor 1254 as washed resting cells and as growing cells with biphenyl as the substrate. Growing cells were far superior to resting-cell suspensions in terms of total polychlorinated biphenyl (PCB) transformation, transformation of specific PCB congeners, and diversity of congeners that were attacked. Growing cells of Acinetobacter sp. strain P6 and Arthrobacter sp. strain B1B transformed 32 and 23% of the [14C]Aroclor 1254, respectively, whereas resting cells of the same respective cultures transformed only 17 and 8%. Transformation was significantly greater with resting cells in only 2 of 39 cases in which congeners were transformed by both growing and resting cells of both cultures. The components of 19 and 12 capillary gas-chromatographic peaks of Aroclor 1254 were transformed by biphenyl-grown resting cells of Acinetobacter sp. strain P6 and Arthrobacter sp. strain B1B, respectively, whereas the components of an additional 6 and 7 peaks were attacked by growing cells of the same respective cultures. Biphenyl oxidation by resting cells of both cultures decreased with time to less than 8% in 28 h. In addition to the normal 2,3-dioxygenase attack on PCBs, Acinetobacter sp. strain P6 also attacked congeners lacking an open 2,3-position. The ability of Acinetobacter sp. strain P6 to transform the components of 25 of the 40 largest peaks of Aroclor 1254 makes it one of the most versatile PCB-transforming organisms yet reported.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed M., Focht D. D. Degradation of polychlorinated biphenyls by two species of Achromobacter. Can J Microbiol. 1973 Jan;19(1):47–52. doi: 10.1139/m73-007. [DOI] [PubMed] [Google Scholar]
- Bedard D. L., Haberl M. L., May R. J., Brennan M. J. Evidence for novel mechanisms of polychlorinated biphenyl metabolism in Alcaligenes eutrophus H850. Appl Environ Microbiol. 1987 May;53(5):1103–1112. doi: 10.1128/aem.53.5.1103-1112.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard D. L., Unterman R., Bopp L. H., Brennan M. J., Haberl M. L., Johnson C. Rapid assay for screening and characterizing microorganisms for the ability to degrade polychlorinated biphenyls. Appl Environ Microbiol. 1986 Apr;51(4):761–768. doi: 10.1128/aem.51.4.761-768.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard D. L., Wagner R. E., Brennan M. J., Haberl M. L., Brown J. F., Jr Extensive degradation of Aroclors and environmentally transformed polychlorinated biphenyls by Alcaligenes eutrophus H850. Appl Environ Microbiol. 1987 May;53(5):1094–1102. doi: 10.1128/aem.53.5.1094-1102.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton H., Stirling D. I. Co-metabolism. Philos Trans R Soc Lond B Biol Sci. 1982 Jun 11;297(1088):481–496. doi: 10.1098/rstb.1982.0056. [DOI] [PubMed] [Google Scholar]
- Furukawa K., Chakrabarty A. M. Involvement of plasmids in total degradation of chlorinated biphenyls. Appl Environ Microbiol. 1982 Sep;44(3):619–626. doi: 10.1128/aem.44.3.619-626.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Tomizuka N., Kamibayashi A. Effect of chlorine substitution on the bacterial metabolism of various polychlorinated biphenyls. Appl Environ Microbiol. 1979 Aug;38(2):301–310. doi: 10.1128/aem.38.2.301-310.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Tomizuka N., Kamibayashi A. Metabolic breakdown of Kaneclors (polychlorobiphenyls) and their products by Acinetobacter sp. Appl Environ Microbiol. 1983 Jul;46(1):140–145. doi: 10.1128/aem.46.1.140-145.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath R. S. Microbial co-metabolism and the degradation of organic compounds in nature. Bacteriol Rev. 1972 Jun;36(2):146–155. doi: 10.1128/br.36.2.146-155.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsuna M., Someda K., Morita K., Yamanouchi Y., Kurimoto T., Kawamura Y., Matsumura H. [Ischemic cerebral symptoms after subarachnoid hemorrhage due to aneurysmal rupture (author's transl)]. No Shinkei Geka. 1978 Jun;6(6):543–548. [PubMed] [Google Scholar]
- Shields M. S., Hooper S. W., Sayler G. S. Plasmid-mediated mineralization of 4-chlorobiphenyl. J Bacteriol. 1985 Sep;163(3):882–889. doi: 10.1128/jb.163.3.882-889.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


