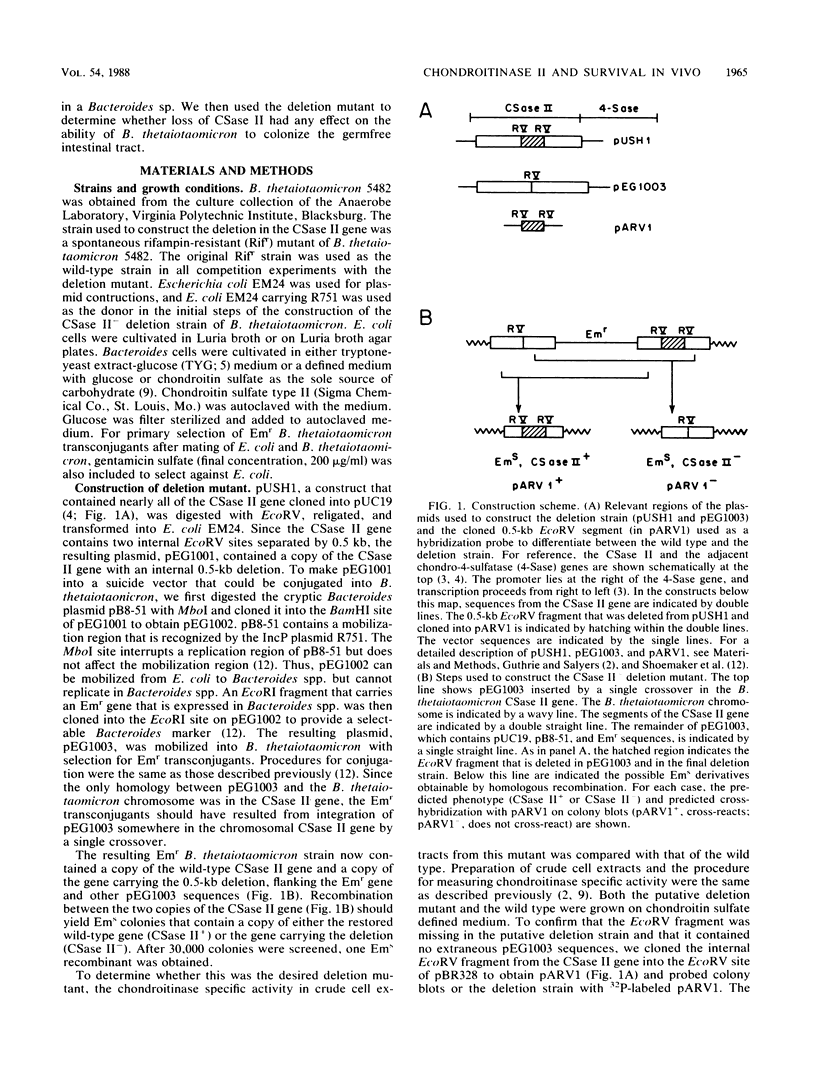
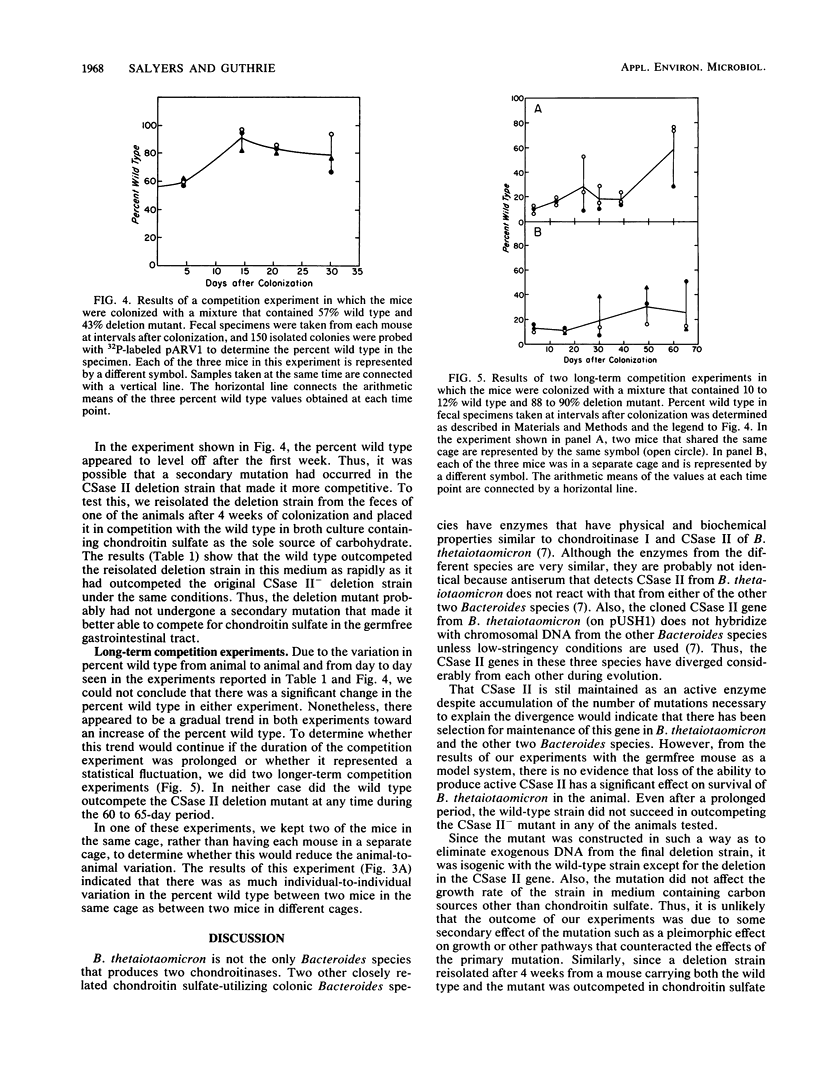
Abstract
Bacteroides thetaiotaomicron, an obligate anaerobe normally found in high concentrations in the human colon, is one of the few colon bacteria that can ferment host mucopolysaccharides such as chondroitin sulfate. Previously, we found that a directed insertional mutation in the gene that codes for the chondroitinase II gene of B. thetaiotaomicron did not affect growth on chondroitin sulfate despite the fact that chondroitinase II accounts for 70% of the total cellular chondroitinase activity. Thus, the chondroitinase II gene did not seem to contribute significantly to growth on chondroitin sulfate when the bacteria were grown in laboratory medium. To determine whether this enzyme is important for bacteria growing in the intestinal tract, we tested the ability of a strain that does not produce chondroitinase II to colonize the intestinal tracts of germfree mice and to compete with wild-type B. thetaiotaomicron. The mutant used in these experiments carried a 0.5-kilobase deletion in the chondroitinase II gene and was constructed so that, unlike the original insertion mutant, it contained no exogenous DNA. The deletion mutant colonized the intestinal tracts of germfree mice at the same levels as the wild type. When a mixture of the deletion mutant and wild type was used to colonize germfree mice, the percent wild type, measured by colony hybridization with the deleted 0.5-kilobase fragment as the hybridization probe, did not rise to 100% even after periods as long as 9 weeks. In most experiments, the percent wild type did not rise significantly above the percent in the original mixture.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Guthrie E. P., Salyers A. A. Evidence that the Bacteroides thetaiotaomicron chondroitin lyase II gene is adjacent to the chondro-4-sulfatase gene and may be part of the same operon. J Bacteriol. 1987 Mar;169(3):1192–1199. doi: 10.1128/jb.169.3.1192-1199.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie E. P., Salyers A. A. Use of targeted insertional mutagenesis to determine whether chondroitin lyase II is essential for chondroitin sulfate utilization by Bacteroides thetaiotaomicron. J Bacteriol. 1986 Jun;166(3):966–971. doi: 10.1128/jb.166.3.966-971.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie E. P., Shoemaker N. B., Salyers A. A. Cloning and expression in Escherichia coli of a gene coding for a chondroitin lyase from Bacteroides thetaiotaomicron. J Bacteriol. 1985 Nov;164(2):510–515. doi: 10.1128/jb.164.2.510-515.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn S., Chan T., Lipeski L., Salyers A. A. Isolation and characterization of two chondroitin lyases from Bacteroides thetaiotaomicron. J Bacteriol. 1983 Nov;156(2):859–866. doi: 10.1128/jb.156.2.859-866.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipeski L., Guthrie E. P., O'Brien M., Kotarski S. F., Salyers A. A. Comparison of proteins involved in chondroitin sulfate utilization by three colonic Bacteroides species. Appl Environ Microbiol. 1986 May;51(5):978–984. doi: 10.1128/aem.51.5.978-984.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., O'Brien M. Cellular location of enzymes involved in chondroitin sulfate breakdown by Bacteroides thetaiotaomicron. J Bacteriol. 1980 Aug;143(2):772–780. doi: 10.1128/jb.143.2.772-780.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., Pajeau M., McCarthy R. E. Importance of mucopolysaccharides as substrates for Bacteroides thetaiotaomicron growing in intestinal tracts of exgermfree mice. Appl Environ Microbiol. 1988 Aug;54(8):1970–1976. doi: 10.1128/aem.54.8.1970-1976.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., Vercellotti J. R., West S. E., Wilkins T. D. Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Appl Environ Microbiol. 1977 Feb;33(2):319–322. doi: 10.1128/aem.33.2.319-322.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Getty C., Guthrie E. P., Salyers A. A. Regions in Bacteroides plasmids pBFTM10 and pB8-51 that allow Escherichia coli-Bacteroides shuttle vectors to be mobilized by IncP plasmids and by a conjugative Bacteroides tetracycline resistance element. J Bacteriol. 1986 Jun;166(3):959–965. doi: 10.1128/jb.166.3.959-965.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitt D. D., Savage D. C. Kinetics of changes induced by indigenous microbiota in the activity levels of alkaline phosphatase and disaccharidases in small intestinal enterocytes in mice. Infect Immun. 1980 Jul;29(1):144–151. doi: 10.1128/iai.29.1.144-151.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]