Abstract
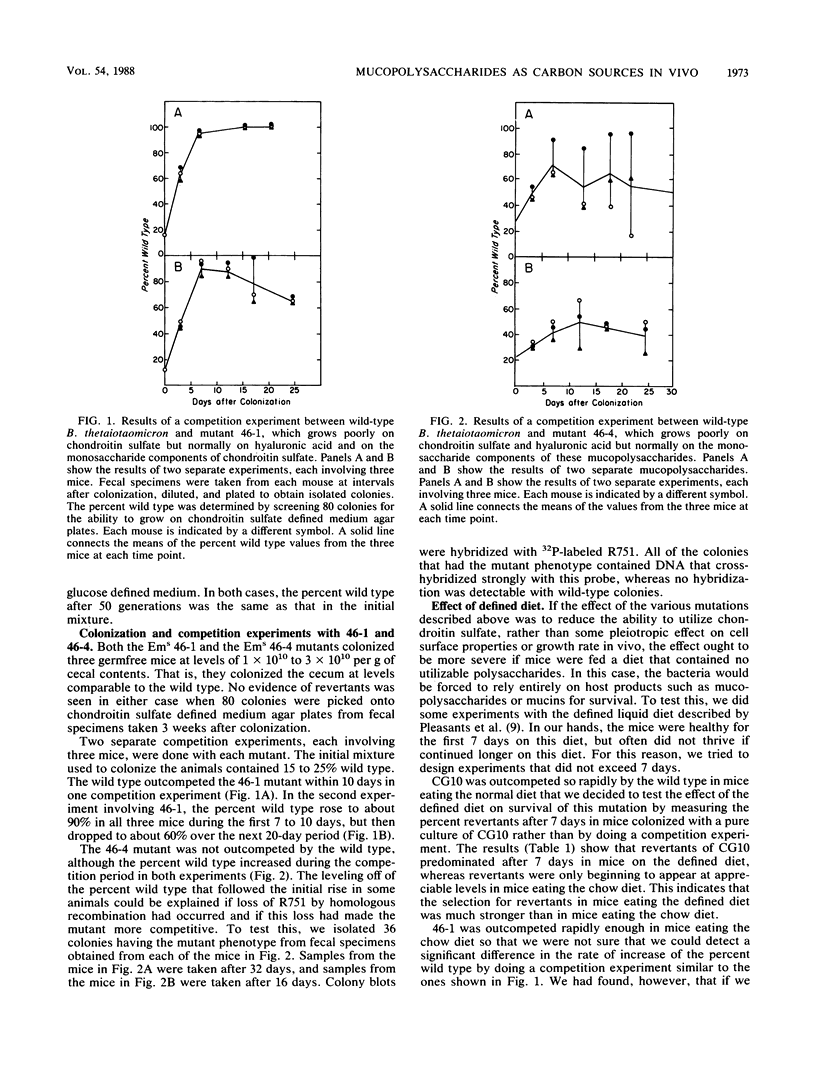
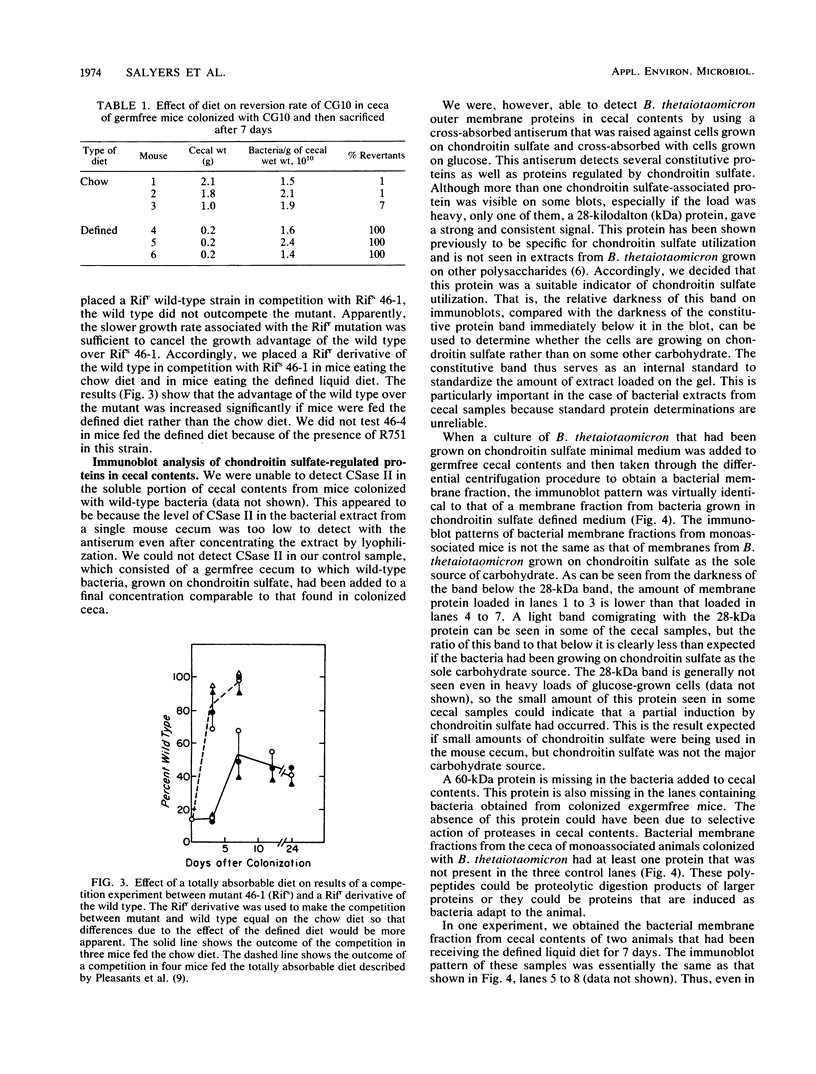
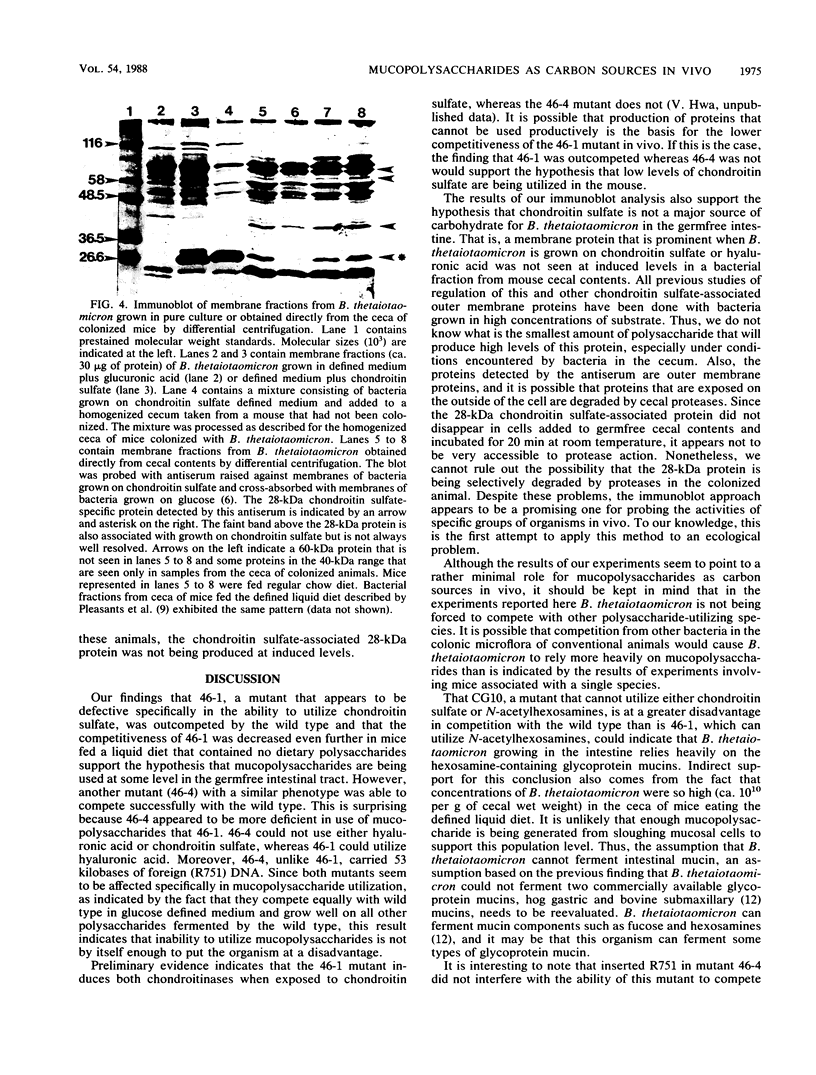
We used two approaches to determine whether the mucopolysaccharide chondroitin sulfate is an important source of carbon and energy for Bacteroides thetaiotaomicron in the intestinal tracts of germfree mice. First, we tested the ability of three mutants that grew poorly or not at all on chondroitin sulfate to colonize the intestinal tract of a germfree mouse and to compete with wild-type B. thetaiotaomicron in this model system. One mutant (CG10) was rapidly outcompeted by the wild type. However, since this mutant was unable to grow on chondroitin sulfate because it could not grow on N-acetyl-galactosamine, one of its monosaccharide components, this mutant might also be unable to utilize glycoprotein mucins. Two mutants (46-1 and 46-4) were isolated that grew poorly on chondroitin sulfate but normally on both component sugars. One of them was outcompeted by the wild type, but the percent wild type increased more slowly than with CG10. In one experiment, the percent wild type never reached 100%. The other (46-4) was not outcompeted by the wild type. These results indicate that, although chondroitin sulfate may be a carbon source in the animal, it is not of major importance. Our second approach was to determine by immunoblot analysis whether a 28-kilodalton outer membrane protein that is produced by B. thetaiotaomicron only when it is grown on chondroitin sulfate or hyaluronic acid was being produced at induced level by B. thetaiotaomicron growing in the ceca of exgermfree mice. There was no evidence for induction of this protein in vivo. Thus, the immunoblot results are consistent with results of the mutant competition experiments.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Guthrie E. P., Salyers A. A. Use of targeted insertional mutagenesis to determine whether chondroitin lyase II is essential for chondroitin sulfate utilization by Bacteroides thetaiotaomicron. J Bacteriol. 1986 Jun;166(3):966–971. doi: 10.1128/jb.166.3.966-971.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie E. P., Shoemaker N. B., Salyers A. A. Cloning and expression in Escherichia coli of a gene coding for a chondroitin lyase from Bacteroides thetaiotaomicron. J Bacteriol. 1985 Nov;164(2):510–515. doi: 10.1128/jb.164.2.510-515.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa V., Shoemaker N. B., Salyers A. A. Direct repeats flanking the Bacteroides transposon Tn4351 are insertion sequence elements. J Bacteriol. 1988 Jan;170(1):449–451. doi: 10.1128/jb.170.1.449-451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotarski S. F., Linz J., Braun D. M., Salyers A. A. Analysis of outer membrane proteins which are associated with growth of Bacteroides thetaiotaomicron on chondroitin sulfate. J Bacteriol. 1985 Sep;163(3):1080–1086. doi: 10.1128/jb.163.3.1080-1086.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn S., Chan T., Lipeski L., Salyers A. A. Isolation and characterization of two chondroitin lyases from Bacteroides thetaiotaomicron. J Bacteriol. 1983 Nov;156(2):859–866. doi: 10.1128/jb.156.2.859-866.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipeski L., Guthrie E. P., O'Brien M., Kotarski S. F., Salyers A. A. Comparison of proteins involved in chondroitin sulfate utilization by three colonic Bacteroides species. Appl Environ Microbiol. 1986 May;51(5):978–984. doi: 10.1128/aem.51.5.978-984.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleasants J. R., Johnson M. H., Wostmann B. S. Adequacy of chemically defined, water-soluble diet for germfree BALB/c mice through successive generations and litters. J Nutr. 1986 Oct;116(10):1949–1964. doi: 10.1093/jn/116.10.1949. [DOI] [PubMed] [Google Scholar]
- Salyers A. A., Guthrie E. P. A deletion in the chromosome of Bacteroides thetaiotaomicron that abolishes production of chondroitinase II does not affect survival of the organism in gastrointestinal tracts of exgermfree mice. Appl Environ Microbiol. 1988 Aug;54(8):1964–1969. doi: 10.1128/aem.54.8.1964-1969.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., O'Brien M. Cellular location of enzymes involved in chondroitin sulfate breakdown by Bacteroides thetaiotaomicron. J Bacteriol. 1980 Aug;143(2):772–780. doi: 10.1128/jb.143.2.772-780.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., Vercellotti J. R., West S. E., Wilkins T. D. Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Appl Environ Microbiol. 1977 Feb;33(2):319–322. doi: 10.1128/aem.33.2.319-322.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Getty C., Gardner J. F., Salyers A. A. Tn4351 transposes in Bacteroides spp. and mediates the integration of plasmid R751 into the Bacteroides chromosome. J Bacteriol. 1986 Mar;165(3):929–936. doi: 10.1128/jb.165.3.929-936.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Salyers A. A. Facilitated transfer of IncP beta R751 derivatives from the chromosome of Bacteroides uniformis to Escherichia coli recipients by a conjugative Bacteroides tetracycline resistance element. J Bacteriol. 1987 Jul;169(7):3160–3167. doi: 10.1128/jb.169.7.3160-3167.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tassell R. L., Wilkins T. D. Isolation of auxotrophs of Bacteroides fragilis. Can J Microbiol. 1978 Dec;24(12):1619–1621. doi: 10.1139/m78-260. [DOI] [PubMed] [Google Scholar]
- Vercellotti J. R., Salyers A. A., Bullard W. S., Wilkins D. Breakdown of mucin and plant polysaccharides in the human colon. Can J Biochem. 1977 Nov;55(11):1190–1196. doi: 10.1139/o77-178. [DOI] [PubMed] [Google Scholar]
- Whitt D. D., Savage D. C. Kinetics of changes induced by indigenous microbiota in the activity levels of alkaline phosphatase and disaccharidases in small intestinal enterocytes in mice. Infect Immun. 1980 Jul;29(1):144–151. doi: 10.1128/iai.29.1.144-151.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]