Abstract
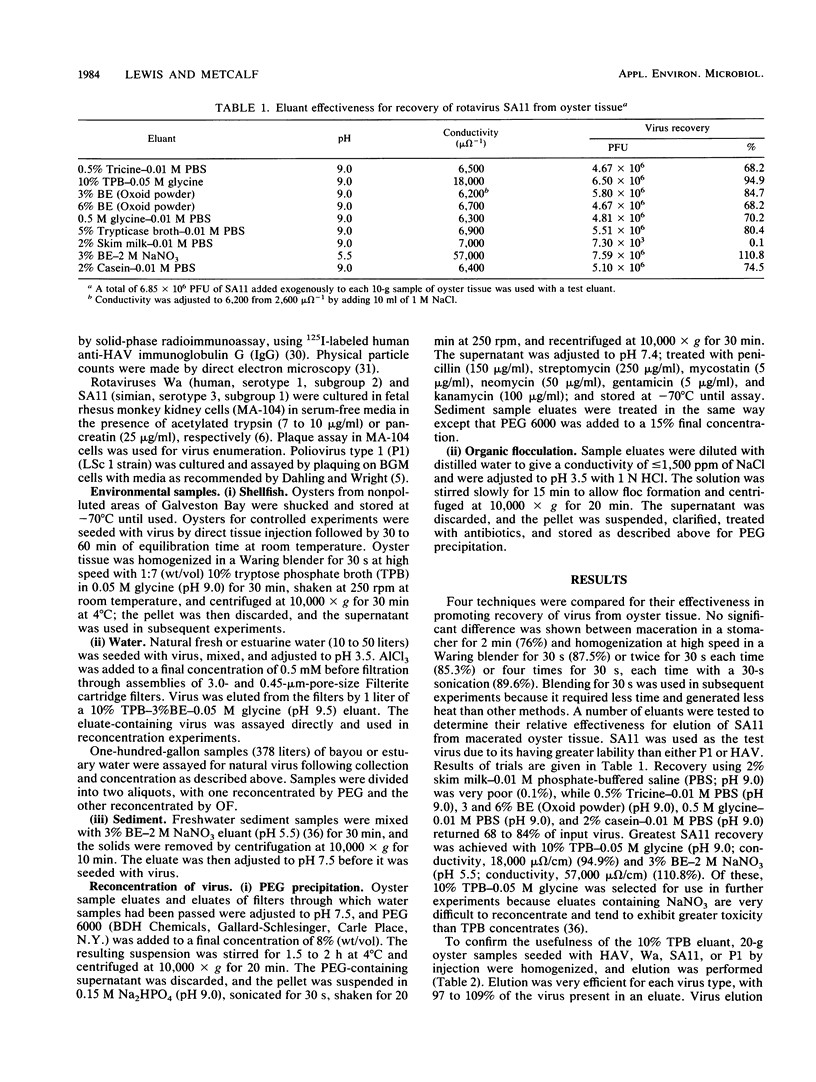
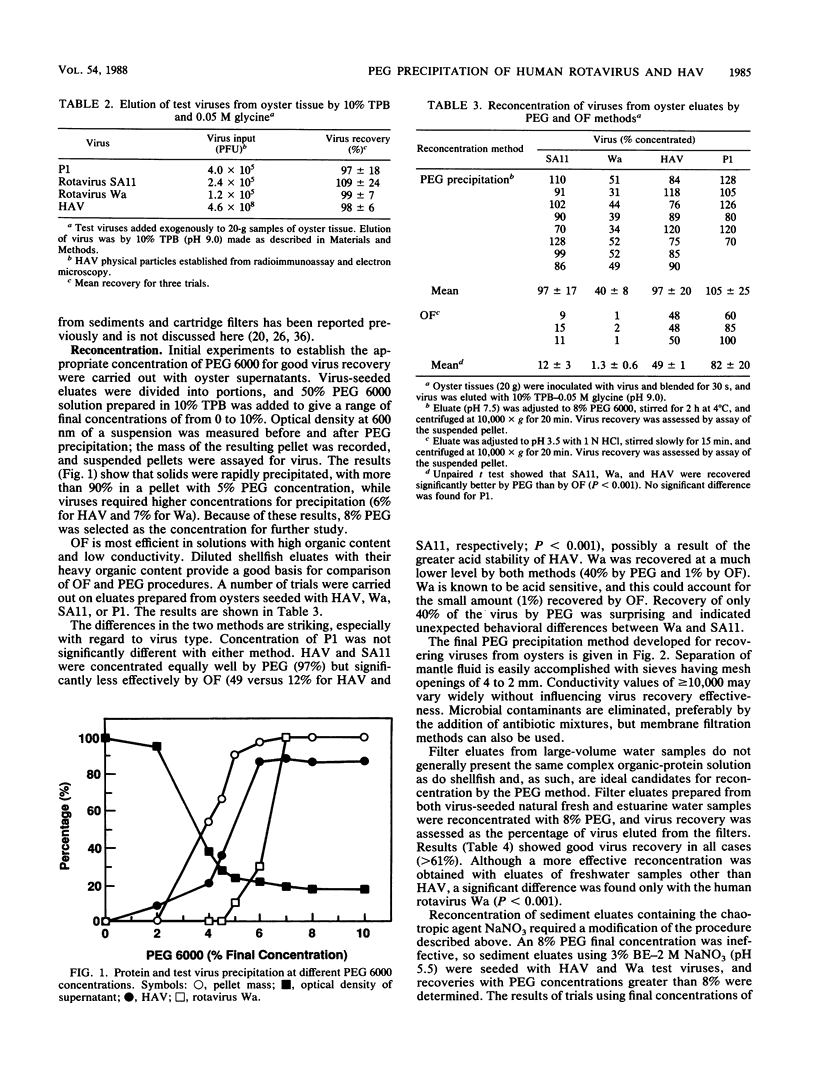
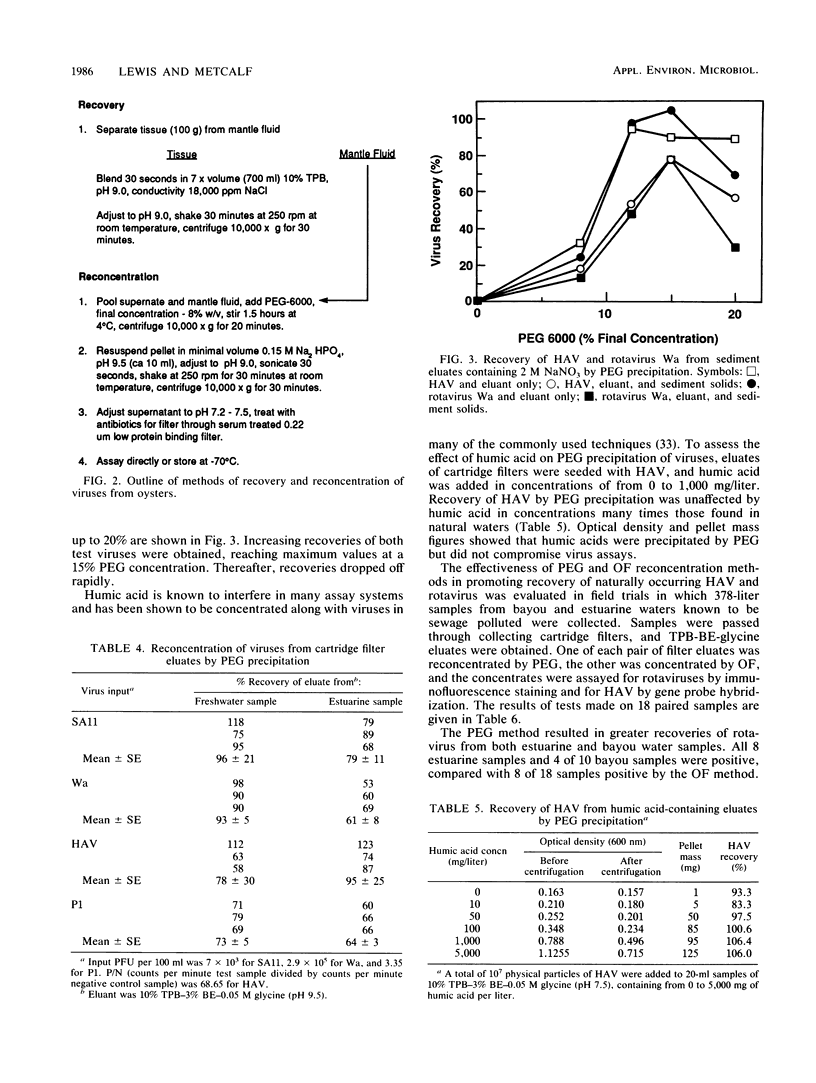
Polyethylene glycol 6000 precipitation was found to be an effective concentration method that enhanced the chances for detecting human virus pathogens in environmental samples. Percent recoveries from eluates of fresh and estuarine waters with 8% polyethylene glycol 6000 averaged 86 for hepatitis A virus, 77 for human rotavirus Wa, 87 for simian rotavirus SA11, and 68 for poliovirus. Percent recoveries of 97, 40, 97 and 105, respectively, for the same viruses were obtained from oyster eluates by the same procedure. Percent recoveries of 97 for hepatitis A virus and 78 for human rotavirus Wa were obtained from sediment eluates containing 2 M NaNO3 with a final concentration of 15% polyethylene glycol 6000. The polyethylene glycol method was shown to be more effective than the organic flocculation method for recovery of hepatitis A virus and rotaviruses Wa and SA11, but not of poliovirus 1 in laboratory studies. In field trials, hepatitis A virus or rotavirus or both were recovered from 12 of 18 eluates by polyethylene glycol, compared with recovery from 9 of 18 eluates by organic flocculation from fresh and estuarine waters subject to pollution.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A. Concentration of Epstein-Barr virus from cell culture fluids with polyethylene glycol. J Gen Virol. 1973 Sep;20(3):391–394. doi: 10.1099/0022-1317-20-3-391. [DOI] [PubMed] [Google Scholar]
- Atha D. H., Ingham K. C. Mechanism of precipitation of proteins by polyethylene glycols. Analysis in terms of excluded volume. J Biol Chem. 1981 Dec 10;256(23):12108–12117. [PubMed] [Google Scholar]
- Bronson D. L., Elliott A. Y., Ritzi D. Concentration of Rous sarcoma virus from tissue culture fluids with polyethylene glycol. Appl Microbiol. 1975 Sep;30(3):464–471. doi: 10.1128/am.30.3.464-471.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahling D. R., Wright B. A. Optimization of the BGM cell line culture and viral assay procedures for monitoring viruses in the environment. Appl Environ Microbiol. 1986 Apr;51(4):790–812. doi: 10.1128/aem.51.4.790-812.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Graham D. Y., Smith E. M., Gerba C. P. Rotavirus stability and inactivation. J Gen Virol. 1979 May;43(2):403–409. doi: 10.1099/0022-1317-43-2-403. [DOI] [PubMed] [Google Scholar]
- Friedmann T., Haas M. Rapid concentration and purification of polyoma virus and SV40 with polyethylene glycol. Virology. 1970 Sep;42(1):248–250. doi: 10.1016/0042-6822(70)90263-1. [DOI] [PubMed] [Google Scholar]
- Heyward J. T., Klimas R. A., Stapp M. D., Obijeski J. F. The rapid concentration and purification of influenza virus from allantoic fluid. Arch Virol. 1977;55(1-2):107–119. doi: 10.1007/BF01314484. [DOI] [PubMed] [Google Scholar]
- Jiang X., Estes M. K., Metcalf T. G., Melnick J. L. Detection of hepatitis A virus in seeded estuarine samples by hybridization with cDNA probes. Appl Environ Microbiol. 1986 Oct;52(4):711–717. doi: 10.1128/aem.52.4.711-717.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanarek A. D., Tribe G. W. Concentration of certain myxoviruses with polyethylene glycol. Nature. 1967 May 27;214(5091):927–928. doi: 10.1038/214927a0. [DOI] [PubMed] [Google Scholar]
- Katzenelson E., Fattal B., Hostovesky T. Organic flocculation: an efficient second-step concentration method for the detection of viruses in tap water. Appl Environ Microbiol. 1976 Oct;32(4):638–639. doi: 10.1128/aem.32.4.638-639.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leberman R. The isolation of plant viruses by means of "simple" coacervates. Virology. 1966 Nov;30(3):341–347. doi: 10.1016/0042-6822(66)90112-7. [DOI] [PubMed] [Google Scholar]
- Lee J. C., Lee L. L. Preferential solvent interactions between proteins and polyethylene glycols. J Biol Chem. 1981 Jan 25;256(2):625–631. [PubMed] [Google Scholar]
- Lewis G. D., Loutit M. W., Austin F. J. A method for detecting human enteroviruses in aquatic sediments. J Virol Methods. 1985 Feb;10(2):153–162. doi: 10.1016/0166-0934(85)90101-6. [DOI] [PubMed] [Google Scholar]
- Lund E., Hedström C. E. The use of an aqueous polymer phase system for enterovirus isolations from sewage. Am J Epidemiol. 1966 Sep;84(2):287–291. doi: 10.1093/oxfordjournals.aje.a120642. [DOI] [PubMed] [Google Scholar]
- McSharry J., Benzinger R. Concentration and purification of vesicular stomatitis virus by polyethylene glycol "precipitation". Virology. 1970 Mar;40(3):745–746. doi: 10.1016/0042-6822(70)90219-9. [DOI] [PubMed] [Google Scholar]
- Metcalf T. G., Melnick J. L. Simple apparatus for collecting estuarine sediments and suspended solids to detect solids-associated virus. Appl Environ Microbiol. 1983 Jan;45(1):323–327. doi: 10.1128/aem.45.1.323-327.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf T. G., Moulton E., Eckerson D. Improved method and test strategy for recovery of enteric viruses from shellfish. Appl Environ Microbiol. 1980 Jan;39(1):141–152. doi: 10.1128/aem.39.1.141-152.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasser A. M., Metcalf T. G. An A-ELISA to detect hepatitis A virus in estuarine samples. Appl Environ Microbiol. 1987 May;53(5):1192–1195. doi: 10.1128/aem.53.5.1192-1195.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILIPSON L., ALBERTSSON P. A., FRICK G. The purification and concentration of viruses by aqueous polymerphase systems. Virology. 1960 Jul;11:553–571. doi: 10.1016/0042-6822(60)90100-8. [DOI] [PubMed] [Google Scholar]
- POLSON A., POTGIETER G. M., LARGIER J. F., MEARS G. E., JOUBERT F. J. THE FRACTIONATION OF PROTEIN MIXTURES BY LINEAR POLYMERS OF HIGH MOLECULAR WEIGHT. Biochim Biophys Acta. 1964 Mar 16;82:463–475. doi: 10.1016/0304-4165(64)90438-6. [DOI] [PubMed] [Google Scholar]
- Rao V. C., Metcalf T. G., Melnick J. L. Development of a method for concentration of rotavirus and its application to recovery of rotaviruses from estuarine waters. Appl Environ Microbiol. 1986 Sep;52(3):484–488. doi: 10.1128/aem.52.3.484-488.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards G. P., Weinheimer D. A. Influence of adsorption time, rocking, and soluble proteins on the plaque assay of monodispersed poliovirus. Appl Environ Microbiol. 1985 Apr;49(4):744–748. doi: 10.1128/aem.49.4.744-748.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortridge K. F., Alexander D. J., Collins M. S. Isolation and properties of viruses from poultry in Hong Kong which represent a new (sixth) distinct group of avian paramyxoviruses. J Gen Virol. 1980 Aug;49(2):255–262. doi: 10.1099/0022-1317-49-2-255. [DOI] [PubMed] [Google Scholar]
- Simmonds R. S., Szücs G., Metcalf T. G., Melnick J. L. Persistently infected cultures as a source of hepatitis A virus. Appl Environ Microbiol. 1985 Apr;49(4):749–755. doi: 10.1128/aem.49.4.749-755.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobsey M. D., Carrick R. J., Jensen H. R. Improved methods for detecting enteric viruses in oysters. Appl Environ Microbiol. 1978 Jul;36(1):121–128. doi: 10.1128/aem.36.1.121-128.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobsey M. D., Hickey A. R. Effects of humic and fulvic acids on poliovirus concentration from water by microporous filtration. Appl Environ Microbiol. 1985 Feb;49(2):259–264. doi: 10.1128/aem.49.2.259-264.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn J. M., Landry E. F., Vicale T. J., Dahl M. C. Modified procedure for the recovery of naturally accumulated poliovirus from oysters. Appl Environ Microbiol. 1979 Oct;38(4):594–598. doi: 10.1128/aem.38.4.594-598.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G. G., Card J. L., Cowan K. M. Immunochemical studies of foot-and-mouth disease. VII. Characterization of foot-and-mouth disease virus concentrated by polyethylene glycol precipitation. Arch Gesamte Virusforsch. 1970;30(4):343–352. doi: 10.1007/BF01258364. [DOI] [PubMed] [Google Scholar]
- Wait D. A., Sobsey M. D. Method for recovery of enteric viruses from estuarine sediments with chaotropic agents. Appl Environ Microbiol. 1983 Aug;46(2):379–385. doi: 10.1128/aem.46.2.379-385.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]


