Abstract
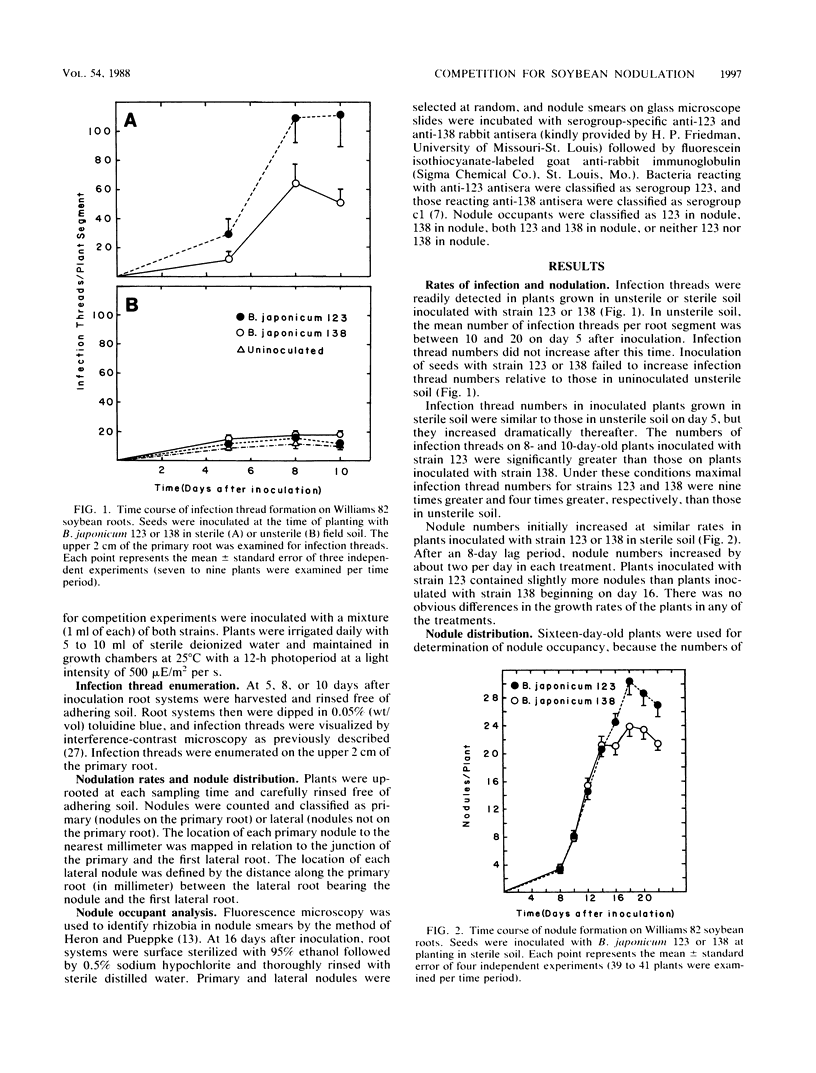
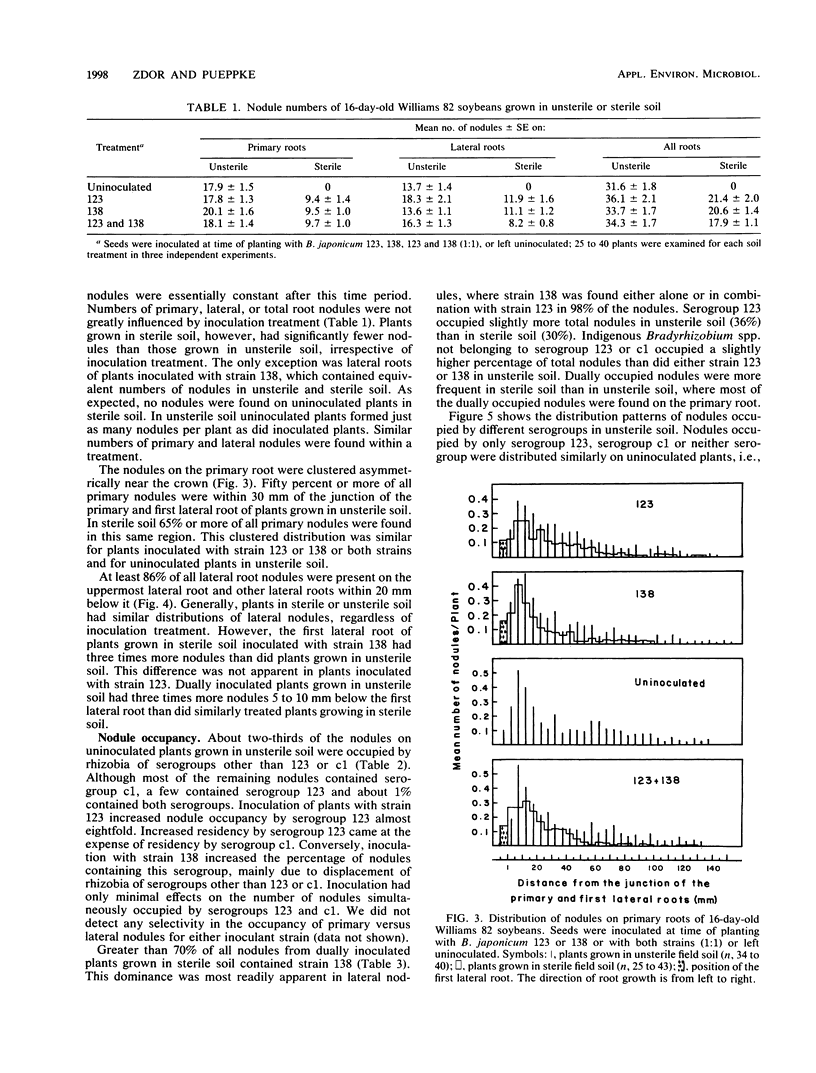
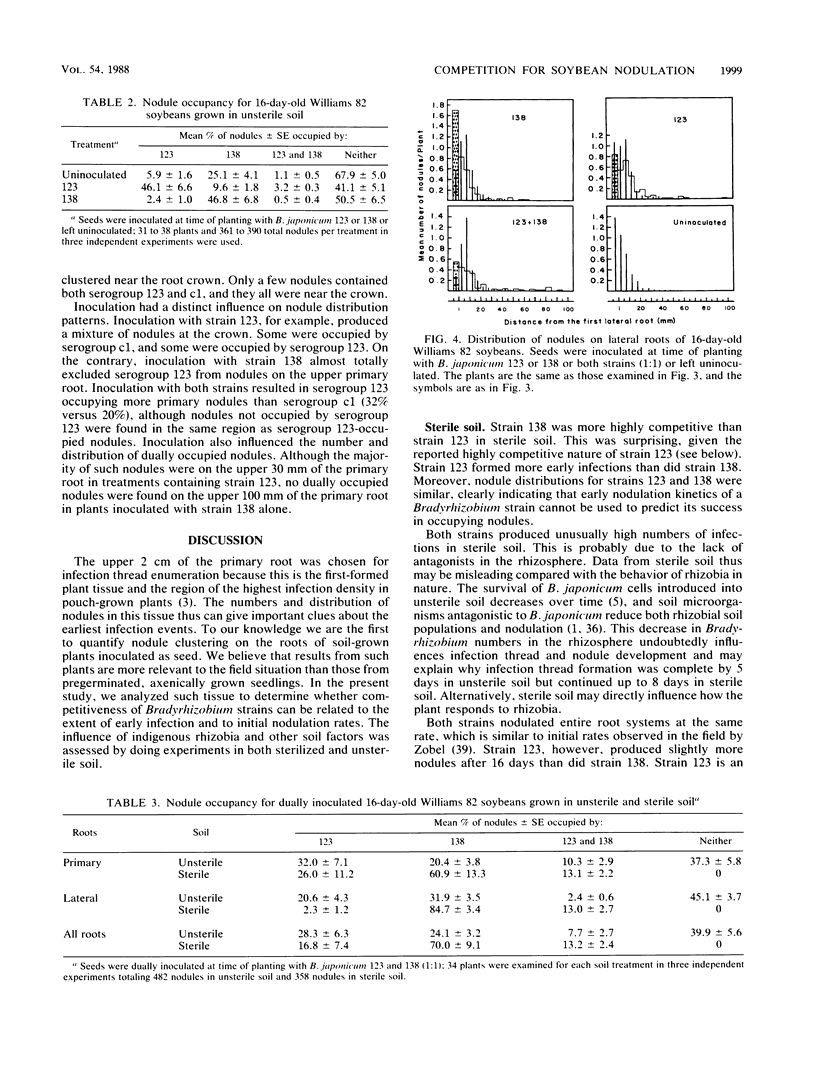
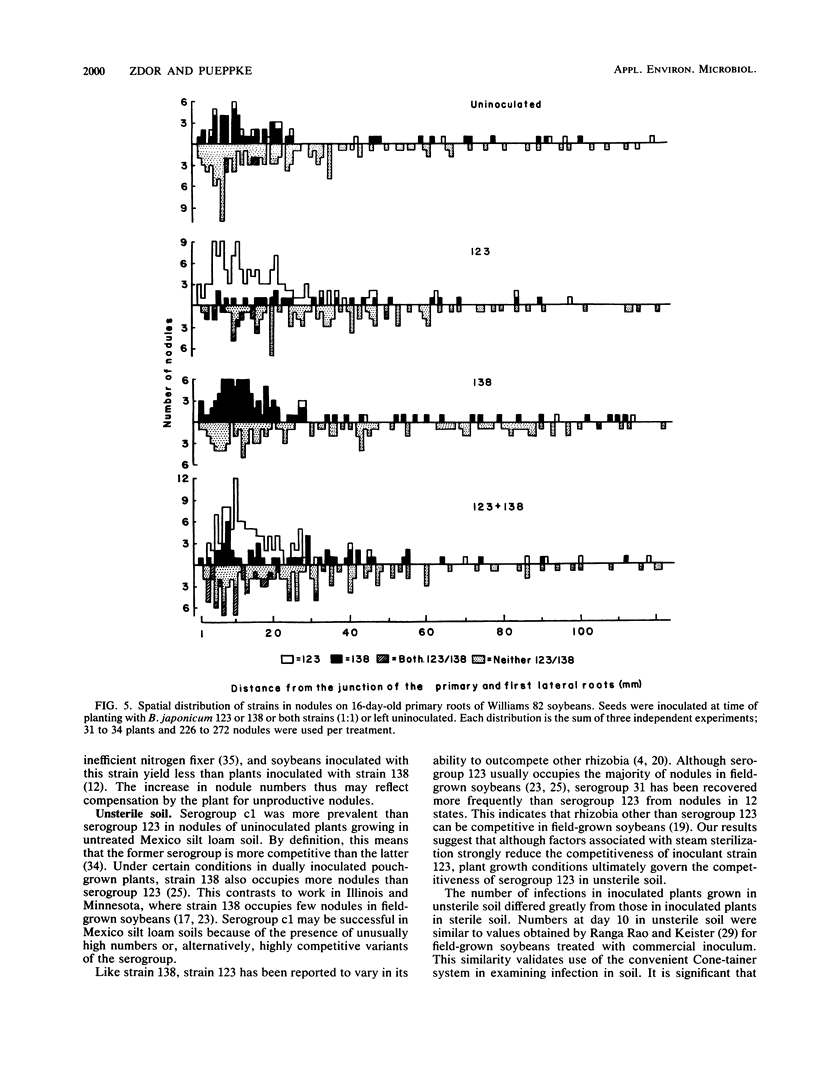
Interactions of soybean with Bradyrhizobium japonicum 123 (serogroup 123) and 138 (serogroup c1) were used to examine the relationship between early infection rates, competition for nodulation, and patterns of nodule occupancy. Both strains formed more infections in autoclaved soil (sterile soil) than in untreated soil (unsterile soil). Inoculation did not increase numbers of infection threads in unsterile soil-grown plants, where infection of proximal portions of primary roots was complete by 5 days after planting. Both strains infected and nodulated at similar rates in sterile soil. Nodules were always clustered on the upper root system, regardless of inoculation and soil treatment. Sixty-seven percent of the nodules of uninoculated plants grown in unsterile soil were occupied by rhizobia belonging to serogroups other than 123 or c1. Inoculation with strain 123 or 138 increased occupancy by that strain at the expense of residency by other rhizobia. Eighty-three percent of all nodules on plants dually inoculated with both strains in sterile soil contained strain 138. The corresponding value for plants inoculated in unsterile soil was 31%. Neither inoculum strain dominated occupancy of first-formed nodules in unsterile soil. It appears that north central Missouri soil may not have populations of highly competitive serogroup 123 and that early infection and nodulation rates do not contribute to the competitive success of strain 138.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhuvaneswari T. V., Turgeon B. G., Bauer W. D. Early Events in the Infection of Soybean (Glycine max L. Merr) by Rhizobium japonicum: I. LOCALIZATION OF INFECTIBLE ROOT CELLS. Plant Physiol. 1980 Dec;66(6):1027–1031. doi: 10.1104/pp.66.6.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DATE R. A., DECKER A. M. MINIMAL ANTIGENIC CONSTITUTION OF 28 STRAINS OF RHIZOBIUM JAPONICUM. Can J Microbiol. 1965 Feb;11:1–8. doi: 10.1139/m65-001. [DOI] [PubMed] [Google Scholar]
- Djordjevic M. A., Redmond J. W., Batley M., Rolfe B. G. Clovers secrete specific phenolic compounds which either stimulate or repress nod gene expression in Rhizobium trifolii. EMBO J. 1987 May;6(5):1173–1179. doi: 10.1002/j.1460-2075.1987.tb02351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron D. S., Pueppke S. G. Regulation of nodulation in the soybean-Rhizobium symbiosis : strain and cultivar variability. Plant Physiol. 1987 Aug;84(4):1391–1396. doi: 10.1104/pp.84.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamicker B. J., Brill W. J. Identification of Bradyrhizobium japonicum Nodule Isolates from Wisconsin Soybean Farms. Appl Environ Microbiol. 1986 Mar;51(3):487–492. doi: 10.1128/aem.51.3.487-492.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyser H. H., Cregan P. B. Nodulation and Competition for Nodulation of Selected Soybean Genotypes among Bradyrhizobium japonicum Serogroup 123 Isolates. Appl Environ Microbiol. 1987 Nov;53(11):2631–2635. doi: 10.1128/aem.53.11.2631-2635.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyser H. H., Weber D. F., Uratsu S. L. Rhizobium japonicum Serogroup and Hydrogenase Phenotype Distribution in 12 States. Appl Environ Microbiol. 1984 Apr;47(4):613–615. doi: 10.1128/aem.47.4.613-615.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslak R. M., Bohlool B. B., Dowdle S., Sadowsky M. J. Competition of Rhizobium japonicum Strains in Early Stages of Soybean Nodulation. Appl Environ Microbiol. 1983 Oct;46(4):870–873. doi: 10.1128/aem.46.4.870-873.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslak R. M., Bohlool B. B. Influence of Environmental Factors on Interstrain Competition in Rhizobium japonicum. Appl Environ Microbiol. 1985 May;49(5):1128–1133. doi: 10.1128/aem.49.5.1128-1133.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslak R. M., Bookland R., Barkei J., Paaren H. E., Appelbaum E. R. Induction of Bradyrhizobium japonicum common nod genes by isoflavones isolated from Glycine max. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7428–7432. doi: 10.1073/pnas.84.21.7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moawad H. A., Ellis W. R., Schmidt E. L. Rhizosphere Response as a Factor in Competition Among Three Serogroups of Indigenous Rhizobium japonicum for Nodulation of Field-Grown Soybeans. Appl Environ Microbiol. 1984 Apr;47(4):607–612. doi: 10.1128/aem.47.4.607-612.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowsky M. J., Tully R. E., Cregan P. B., Keyser H. H. Genetic Diversity in Bradyrhizobium japonicum Serogroup 123 and Its Relation to Genotype-Specific Nodulation of Soybean. Appl Environ Microbiol. 1987 Nov;53(11):2624–2630. doi: 10.1128/aem.53.11.2624-2630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E. L., Zidwick M. J., Abebe H. M. Bradyrhizobium japonicum Serocluster 123 and Diversity among Member Isolates. Appl Environ Microbiol. 1986 Jun;51(6):1212–1215. doi: 10.1128/aem.51.6.1212-1215.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield P. R., Gibson A. H., Dudman W. F., Watson J. M. Evidence for genetic exchange and recombination of Rhizobium symbiotic plasmids in a soil population. Appl Environ Microbiol. 1987 Dec;53(12):2942–2947. doi: 10.1128/aem.53.12.2942-2947.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viteri S. E., Schmidt E. L. Ecology of Indigenous Soil Rhizobia: Response of Bradyrhizobium japonicum to Readily Available Substrates. Appl Environ Microbiol. 1987 Aug;53(8):1872–1875. doi: 10.1128/aem.53.8.1872-1875.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


