Abstract
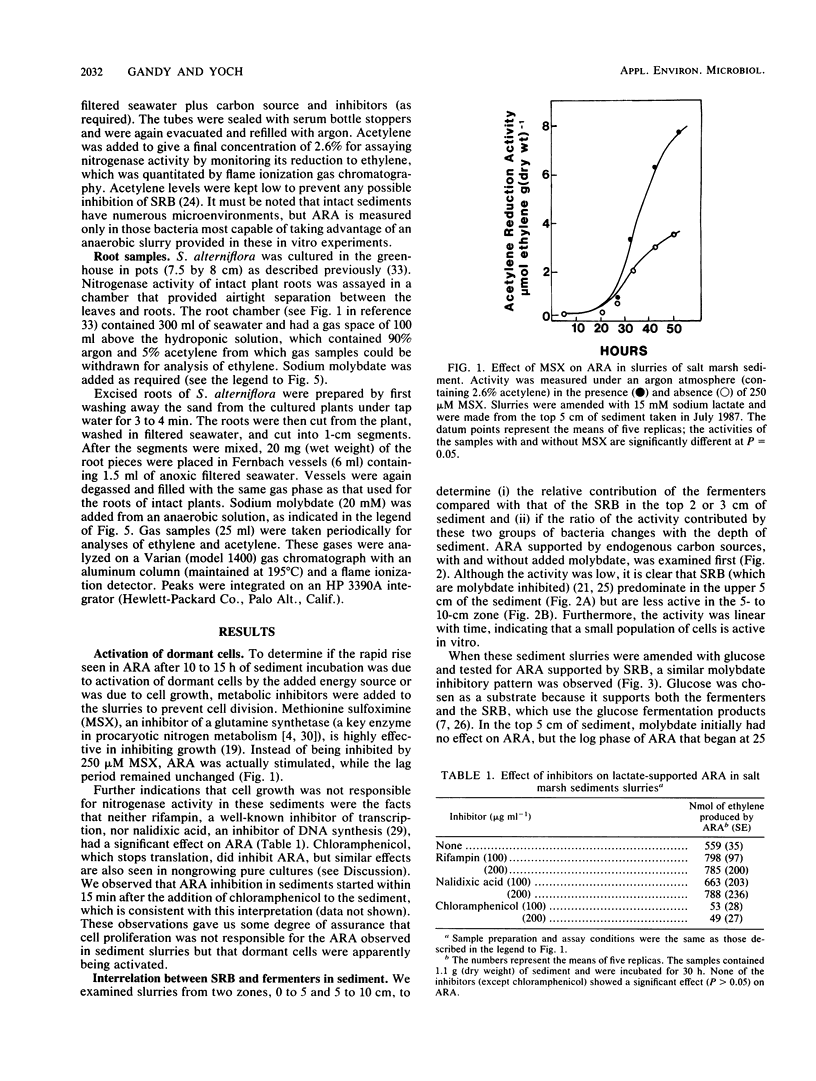
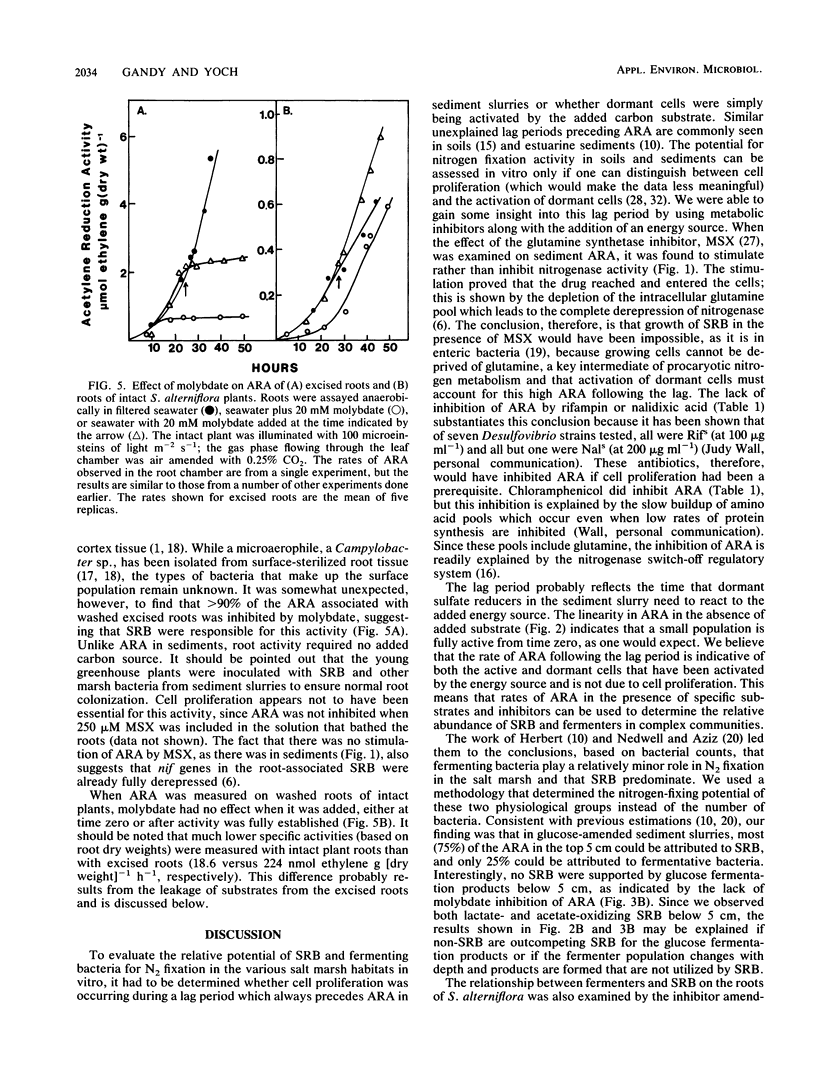
A combination of inhibitors and carbon substrates was used to determine the relative contribution of sulfate-reducing bacteria (SRB) and fermenting bacteria to nitrogen fixation in a salt marsh sediment and on the roots of Spartina alterniflora. Because a lag period precedes acetylene-reducing activity (ARA) in amended sediments, an extensive analysis was done to be sure that this activity was due to the activation of dormant cells, not simply to cell proliferation. Since ARA was not affected by metabolic inhibitors such as rifampin, nalidixic acid, or methionine sulfoximine, it appeared that cell growth was not responsible for this activity. Instead, dormant cells were being activated by the added energy source. Molybdate inhibition studies with glucose-amended sediment slurries indicated that ARA in the upper 5 cm of the salt marsh was due primarily (70%) to SRB and that below that level (5 to 10 cm) it was due primarily (greater than 90%) to fermenting bacteria. ARA associated with washed roots of intact S. alterniflora plants was not inhibited by molybdate, which indicates that bacteria other than SRB were responsible. However, when the roots were excised from the plant, the activity (per unit of root mass) was 10-fold higher and was severely inhibited by molybdate. While this high activity is probably an artifact, due to the release of oxidizable substrates from the excised roots, it indicates that SRB are present in high numbers on Spartina roots.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyle C. D., Patriquin D. G. Endorhizal and Exorhizal Acetylene-reducing Activity in a Grass (Spartina alterniflora Loisel.)-Diazotroph Association. Plant Physiol. 1980 Aug;66(2):276–280. doi: 10.1104/pp.66.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker H. J., Smith D. W. Enumeration and relative importance of acetylene-reducing (nitrogen-fixing) bacteria in a delaware salt marsh. Appl Environ Microbiol. 1980 May;39(5):1019–1025. doi: 10.1128/aem.39.5.1019-1025.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. K., Brill W. J. Derepression of nitrogenase synthesis in the presence of excess NH4+. Biochem Biophys Res Commun. 1974 Aug 5;59(3):967–971. doi: 10.1016/s0006-291x(74)80074-4. [DOI] [PubMed] [Google Scholar]
- Hanson R. B. Comparison of Nitrogen Fixation Activity in Tall and Short Spartina alterniflora Salt Marsh Soils. Appl Environ Microbiol. 1977 Mar;33(3):596–602. doi: 10.1128/aem.33.3.596-602.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar H. F., Wuhrmann K. Kinetic parameters and relative turnovers of some important catabolic reactions in digesting sludge. Appl Environ Microbiol. 1978 Jul;36(1):1–7. doi: 10.1128/aem.36.1.1-7.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. M., Klug M. J. Glucose metabolism in sediments of a eutrophic lake: tracer analysis of uptake and product formation. Appl Environ Microbiol. 1982 Dec;44(6):1308–1317. doi: 10.1128/aem.44.6.1308-1317.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. D., Hu C. Z., Yoch D. C. Changes in amino acid and nucleotide pools of Rhodospirillum rubrum during switch-off of nitrogenase activity initiated by NH4+ or darkness. J Bacteriol. 1987 Jan;169(1):231–237. doi: 10.1128/jb.169.1.231-237.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung C. R., Patriquin D. G. Isolation of a nitrogen-fixing Campylobacter species from the roots of Spartina alterniflora Loisel. Can J Microbiol. 1980 Aug;26(8):881–886. doi: 10.1139/m80-153. [DOI] [PubMed] [Google Scholar]
- McClung C. R., van Berkum P., Davis R. E., Sloger C. Enumeration and Localization of N(2)-Fixing Bacteria Associated with Roots of Spartina alterniflora Loisel. Appl Environ Microbiol. 1983 Jun;45(6):1914–1920. doi: 10.1128/aem.45.6.1914-1920.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. S., Brenchley J. E. L-Methionine SR-sulfoximine-resistant glutamine synthetase from mutants of Salmonella typhimurium. J Biol Chem. 1981 Nov 10;256(21):11307–11312. [PubMed] [Google Scholar]
- PECK H. D., Jr Symposium on metabolism of inorganic compounds. V. Comparative metabolism of inorganic sulfur compounds in microorganisms. Bacteriol Rev. 1962 Mar;26:67–94. doi: 10.1128/br.26.1.67-94.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne W. J., Grant M. A. Influence of acetylene on growth of sulfate-respiring bacteria. Appl Environ Microbiol. 1982 Mar;43(3):727–730. doi: 10.1128/aem.43.3.727-730.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronzio R. A., Rowe W. B., Meister A. Studies on the mechanism of inhibition of glutamine synthetase by methionine sulfoximine. Biochemistry. 1969 Mar;8(3):1066–1075. doi: 10.1021/bi00831a038. [DOI] [PubMed] [Google Scholar]
- Roszak D. B., Colwell R. R. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987 Sep;51(3):365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanzey B. Modulation of gene expression by drugs affecting deoxyribonucleic acid gyrase. J Bacteriol. 1979 Apr;138(1):40–47. doi: 10.1128/jb.138.1.40-47.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting G. J., Gandy E. L., Yoch D. C. Tight coupling of root-associated nitrogen fixation and plant photosynthesis in the salt marsh grass Spartina alterniflora and carbon dioxide enhancement of nitrogenase activity. Appl Environ Microbiol. 1986 Jul;52(1):108–113. doi: 10.1128/aem.52.1.108-113.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdel F., Pfennig N. A new anaerobic, sporing, acetate-oxidizing, sulfate-reducing bacterium, Desulfotomaculum (emend.) acetoxidans. Arch Microbiol. 1977 Feb 4;112(1):119–122. doi: 10.1007/BF00446665. [DOI] [PubMed] [Google Scholar]
- Yoch D. C., Whiting G. J. Evidence for NH4+ switch-off regulation of nitrogenase activity by bacteria in salt marsh sediments and roots of the grass Spartina alterniflora. Appl Environ Microbiol. 1986 Jan;51(1):143–149. doi: 10.1128/aem.51.1.143-149.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berkum P., Sloger C. Immediate acetylene reduction by excised grass roots not previously preincubated at low oxygen tensions. Plant Physiol. 1979 Nov;64(5):739–743. doi: 10.1104/pp.64.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]



