Abstract
The ribonucleoprotein (RNP) enzyme telomerase is required for replication of eukaryotic chromosomal termini. The RNA moiety of telomerase is essential for enzyme function and provides the template for telomeric DNA synthesis. However, the roles of its nontemplate domains have not been explored. Here we demonstrate that a novel interspecies telomerase RNA swap in vivo creates a functional but aberrant telomerase. Telomerase RNA from the ciliate Glaucoma chattoni was expressed in Tetrahymena thermophila cells. The telomerase RNAs from these two species have almost superimposable secondary structures. The template region base sequence is identical in the two RNAs, but elsewhere their sequences differ by 49%. This hybrid telomerase RNP was enzymatically active but added only short stretches of telomeric repeat tracts in vivo and in vitro. This new enzyme also had a strong, aberrant DNA cleavage activity in vitro. Thus, molecular interactions in the RNP involving nontemplate RNA domains affect specific aspects of telomerase enzyme function, raising the possibility that they may regulate telomerase activity.
The G-rich telomeric DNA tracts found at most eukaryotic chromosomal termini are added by a specialized component of the cellular DNA replication machinery, the ribonucleoprotein (RNP) reverse transcriptase telomerase. Telomeric DNA has numerous important functions and properties, including allowing completion of DNA replication, mediating chromosome stability (1), and affecting mitotic chromosome separation (2). Therefore, telomere synthesis and maintenance are crucial aspects of stable genomic inheritance in eukaryotes. The RNA moiety of the telomerase RNP is essential for enzymatic function and contains an internal sequence that templates telomeric DNA polymerization (3). Studies of template point mutations also have revealed other critical roles for specific template residues in enzyme action (4, 5). However, little is known about the function of other portions of telomerase RNA.
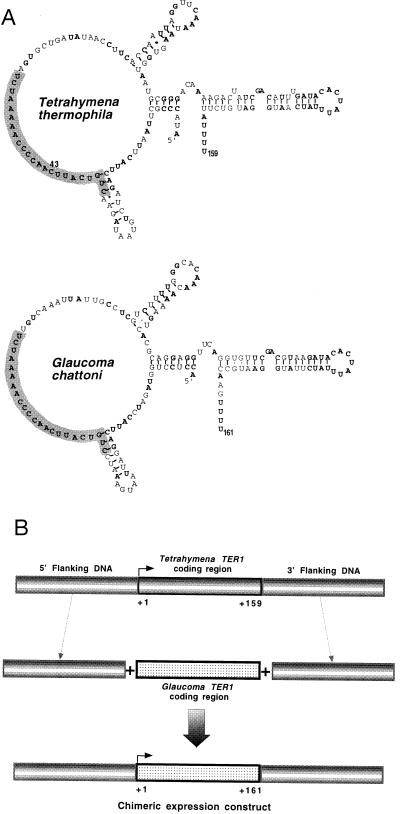
Telomerase RNAs in ciliated protozoa (3, 6, 7), yeasts (8, 9), and mammals (10, 11) have diverged rapidly in primary sequence. Phylogenetic (12–14) and RNA conformational analyses (15, 16) of the small (≈150–200 nt) ciliate telomerase RNAs have shown that their secondary structures are highly conserved. Comparison of 13 telomerase RNAs from one group of ciliates has shown a strikingly bipartite sequence arrangement; they all contain a 17-nt stretch of identical sequence centered on the template domain, and the primary sequence of the rest of the RNA differs by up to 76% yet preserves similar structural domains (12, 14). To investigate directly how this nontemplate portion of telomerase RNA affects enzymatic function, we tested whether the ≈160-nt telomerase RNAs in the ciliates Tetrahymena thermophila and Glaucoma chattoni were functionally interchangeable in vivo. These two telomerase RNAs share an identical 23-base sequence in and around the template domain. In contrast, the rest of the RNA sequence, which has an almost identical secondary structure (15), is 49% different (Fig. 1A).
Figure 1.
Conserved similarities between T. thermophila and G. chattoni telomerase RNAs (TER1). (A) Schematic summary of the conserved secondary structure between Tetrahymena and Glaucoma TER1 RNAs. Residues in bold represent bases conserved between these two RNAs. Shaded region contains 23 bases of absolute sequence identity centered upon the template region. The templating region contains an RNA sequence specifying the complementary telomeric DNA sequence polymerized at chromosome termini. The position of the C-to-A template base change (43A) is indicated. Despite an overall 35% difference in RNA sequence (49% excluding the template region), both TER1 RNAs fold into similar conformations. (B) Schematic strategy for the heterologous expression of the G. chattoni telomerase RNA gene (Gc.TER1) in T. thermophila cells. A PCR approach was devised to precisely surround the Glaucoma TER1 coding region with Tetrahymena TER1 expression signals producing a chimeric Gc.TER1 gene, which was introduced into wild-type T. thermphila cells.
Here we demonstrate that, in a cross-species telomerase RNA swap, the Glaucoma telomerase RNA, despite its ≈50% sequence divergence outside the template region, assembles and functions in vivo in Tetrahymena cells. The resulting hybrid enzyme, containing Glaucoma telomerase RNA and Tetrahymena telomerase proteins, has lower activity and polymerizes repeats less processively than the wild-type Tetrahymena enzyme. Strikingly, the hybrid telomerase activity also displays an aberrant cleavage activity. This study reveals a new class of telomerase RNA mutants, indicating that telomerase RNA domains other than the template affect enzymatic processes taking place at the polymerization active-site.
METHODS
PCR and Transformation.
The chimeric Gc.TER1 gene was constructed as three consecutively overlapping PCR fragments using methods described previously (4, 5). Sequences of oligonucleotide primers used to synthesize the three fragments are indicated.
Upstream.
5′GGGGGGGGGGGGTGATCACTCGAGGGAGCTCATAAAA; 5′GGATCTACCAGGAGGTAAAAGACTTAAAATAATTTCTAC. Coding region: 5′GTAGAAATTATTTTAAGTCTTTTACCTCCTGGTAGATCC; 5′GAATACAAATCGAAATAGATAAAAAAAA CTTGGCATTCCATAAGATAAATAGTG.
Downstream.
5′GGAATGCCAAGTTTTTTTTATCTATTTCGATTTGTATTC; 5′GGGGGGGTACCCTCGAGGGAAGCTATTTTTAG.
The 43A template mutation was introduced into the chimeric Gc.TER1 gene by a similar PCR method using the following primers: 5′CCTGTCATTAAACCCCAAAAATC and 5′GATTTTTGGGGTTTAATGACAGG.
Gc.TER1 and Gc.ter1–43A PCR fragments were cloned into a TA-cloning vector (Invitrogen). Correct sequence of gene inserts was verified (Sequenase, United States Biochemical), and ≈0.5 kb XhoI DNA fragments bearing Gc.TER1 or Gc.ter1–43A genes were subcloned into the high copy, episomal vector prD4–1. Transformation of wild-type T. thermophila cells (B2086 and CU428) was performed using established electroporation methods. Drug selection (100 μg/ml paromomycin) was applied 16 h after electroporation, and transformants were selected 3–4 days later.
Northern Blot Analysis.
Pooled transformants (>15–20 single transformant cell lines) were grown in liquid culture at 30°C with shaking (100 rpm) for 1–2 days. Total RNA was prepared from 50-ml cultures. Total RNA (10–20 μg) was electrophoresed through 5% acrylamide/7 M urea denaturing gels. After transfer to Hybond-N+ membrane (Amersham), blots were probed with random-prime, labeled PCR product for Tt.TER1 or Gc.TER1 gene probes. Primers to generate TER1-specific probes have been described (15). Hybridization was performed using the SDS/Na2HPO4 method in 0.5 M phosphate at 25°C overnight. Tt.TER1-specific probed blots were washed three times at 25°C for 5 min, once at 55°C for 5 min, and once at 25°C for 5 min in 50 mM phosphate buffer. Gc.TER1-specific, probed blots were washed as described above, except once a 60°C, 5-min wash replaced the 55°C wash.
RNP Gel Analysis.
S100 extract was prepared from T. thermophila cells, grown as described above. RNP complexes were analyzed on composite 3.5% polyacrylamide (60:1)/0.6% agarose in 50 mM Tris·acetate (pH 7.5) buffer. Approximately equal amounts of total S100 protein (Bradford, Pierce, IL) were loaded onto gels. RNase sensitivity of RNP complexes was determined by RNase A treatment (50 μg/ml for 5 min at 25°C) before gel loading. After electrophoresis, gels were denatured in 50% urea and transferred to membrane. Gel blots for Western blot analysis were transferred onto poly(vinylidene difluoride) membrane (Millipore).
Western Blot Analysis.
Anti-p80 and -p95 antibodies were a generous gift from Kathleen Collins (University of California, Berkeley, CA). Western blot analysis was performed with an enhanced chemiluminescence detection kit (Amersham) according to the manufacturer’s instructions.
Southern Blot Analysis.
Southern blot detection of contiguous G4T3 and G4T2 telomeric repeat tracts from Glaucoma and Tetrahymena telomerase RNA transformant genomic DNAs (PstI-cut) was performed as described (17). The G4T3 repeat probe used was 5′-TTT(GGGGTTT)3, and the G4T2 probe was 5′-GTT(GGGGTT)3G. TER1 gene dosage studies were determined using duplicate Southern blots of PstI-cut genomic DNA prepared from Tetrahymena and Glaucoma TER1 and ter1–43A transformants probed with Tt.TER1 or Gc.TER1 gene probes. Vector plasmids bearing Tt.TER1 or Gc.TER1 genes provided positive controls for probe hybridization.
Cloning and Screening Transformant Telomeres.
Uncut Tt.ter1–43A or Gc.ter1–43A transformant genomic DNAs (≈1 μg) were T4 DNA polymerase-treated before ligation to EcoRV-cut Bluescript vector (Stratagene). After EcoRI cleavage and religation, approximately one–sixth of both telomere libraries was transformed into Escherichia coli (XL1 Blue MRF’, Stratagene) and plated. Libraries were screened first with the G4T3-specific oligonucleotide probe and reprobed with the G4T2-specific probe. Plasmids containing G4T3 and G4T2 repeat tracts provided positive controls (17). Extent of G4T3 probe hybridization was >10 fold in Tt.ter1–43A telomere clones compared with Gc.ter1–43A telomere clones, likely resulting from better probe hybridization to longer variant repeat tracts present in the Tt.ter1–43A telomeric clones vis-à-vis shorter tracts in Gc.ter1–43A clones. G4T3-positive clones were DNA sequenced using a T7 promoter primer.
Purification of Telomerase Activity.
Extracts prepared from pooled T. thermophila transformant cell lines were purified over heparin–agarose, DEAE–cellulose, and octyl–Sepharose columns as described (4, 5). Labeled dTTP*-only and dGTP* plus dTTP telomerase reactions were performed as described (4) except final primer and nucleotide concentrations were 1 μM and 5 μM, respectively. Reactions were performed typically in a 20-μl volume at 25°C for between 10 and 60 min. In mixing experiments, relative ratios of Tt.TER1:Tt.ter-43A extracts were mixed as follows and were subject to a G + T reaction: 1:0.9, 1:0.7, and 1:0.5. The (T2G4)3 primer was gel purified to nucleotide resolution and heat denatured before use. Labeled reaction products were separated on denaturing gels and visualized by autoradiography.
RESULTS
Heterologous Expression of Glaucoma Telomerase RNA.
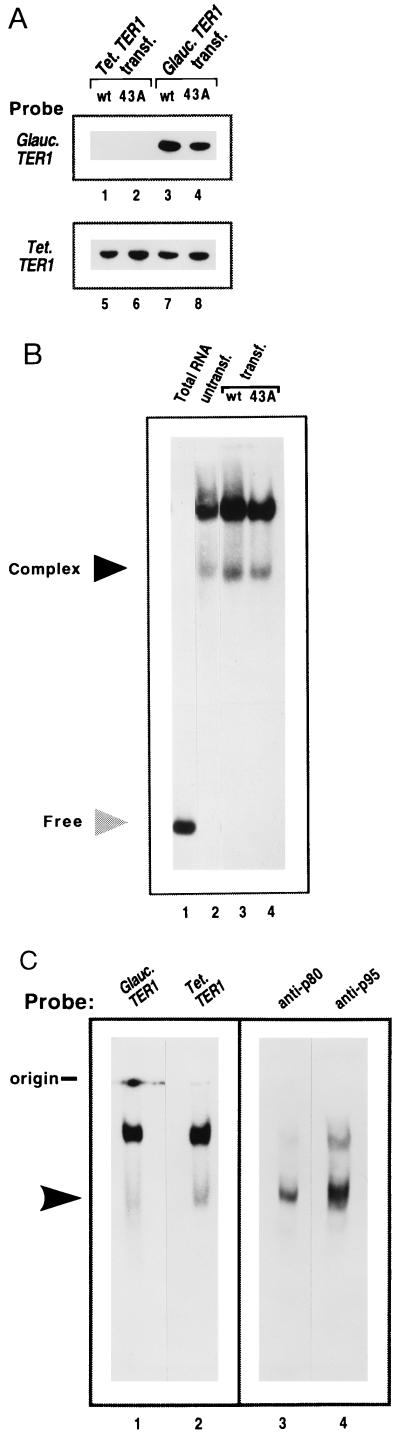
The Glaucoma telomerase RNA gene (Gc.TER1) was expressed on a high copy number vector in Tetrahymena cells (Fig. 1B). Previous work has shown that such an introduced, template-marked T. thermophila telomerase RNA can replace the endogenous telomerase RNA in the telomerase RNP (4, 5). A template mutation was introduced into the Gc.TER1 gene to provide a direct “read-out” of in vivo gene activity distinguishable from the endogenous, wild-type Tetrahymena telomerase activity. The same mutation was a C-to-A substitution at the 5′-most position in the RNA template (43A mutation; Fig. 1A). This mutation in the T. thermophila RNA (Tt.ter1–43A) causes synthesis of G4T3 variant repeats instead of wild-type G4T2 repeats in vitro and in vivo (4). The Gc.TER1 and Gc.ter1–43A constructs, or corresponding Tt.TER1 and Tt.ter1-43A gene constructs as controls (4, 18), were introduced into wild-type T. thermophila cells. Southern and Northern blotting analyses of transformants showed that the Gc.TER1 gene was present at the expected high copy number (data not shown) and was transcribed to produce stable RNA (Fig. 2A). The steady-state levels of Gc.TER1 RNA were similar to those found in the control Tt.TER1 transformants (Fig. 2A, lanes 5 and 6) and were comparable to endogenous Tt.TER1 RNA levels (Fig. 2A, lanes 7 and 8).
Figure 2.
Heterologous expression and assembly of Glaucoma telomerase RNA into an RNP complex. (A) Northern blot analysis of Tetrahymena transformants expressing the Gc.TER1 gene in vivo. Total RNA from Tetrahymena or Glaucoma TER1 or ter1–43A transformants was fractionated on denaturing gels. Blots probed with a Gc.TER1-specific gene probe (lanes 1–4) revealed presence of Gc.TER1 RNA in vivo (lanes 3 and 4). Blots were reprobed with a Tt.TER1-specific probe (lanes 5–8); endogenous Tetrahymena TER1 RNA was observed in Gc.TER1 and Gc.ter1–43A transformants (lanes 7 and 8). (B) Assembly of Tetrahymena TER1 RNA into a telomerase RNP complex. RNP complexes prepared from untransformed, vegetative Tetrahymena cells (lane 2) and Tt.TER1 or Tt.ter1–43A transformants (lanes 3 and 4) were studied by RNP gel analysis; blots were probed with a Tetrahymena TER1-specific probe (see Methods). Lane 1 contains total RNA (≈10 μg) prepared from Tt.TER1 transformants. Light shaded arrow indicates free telomerase RNA, and dark arrow indicates lower putative telomerase RNP complex. (C) Identity of RNP complexes in Gc.ter1–43A transformants. RNP complexes from Gc.ter1–43A transformants were analyzed by sequentially probing with a Gc.TER1-specific probe (lane 1) and then a Tt.TER1-specific probe (lane 2). Lanes 3 and 4 are duplicate loadings subjected to Western blot analysis using telomerase anti-p80 and -p95 antibodies, respectively. Arrow identifies lower RNP complex.
Telomerase RNP Assembly.
Telomerase RNP formation was assessed by nondenaturing gel electrophoretic analysis of S100 fractions prepared from transformed cells or untransformed control cells (Fig. 2 B, lanes 2–4, and C, lane 2). A Tt.TER1-specific probe identified two RNP complexes both sensitive to RNase A pretreatment that were similar in all of these cell lines. In analyses of the Gc.ter1–43A S100 extracts, sequential probing for either the Glaucoma or the Tetrahymena TER1 RNA showed that comparable levels of both RNAs were present in the RNP complexes (Fig. 2C, lanes 1 and 2). Western blotting analysis of these same RNP complexes, using separate antibodies specific for the T. thermophila telomerase 80- and 95-kDa protein components (19), showed that the majority of signal for both telomerase proteins was in the lower RNP complex (Fig. 2C, lanes 3 and 4, arrow). These results indicated that both Tetrahymena telomerase p80/p95 proteins and Glaucoma telomerase RNA were present in the lower RNP complex.
Thus, despite its 49% sequence difference outside the template region, the Glaucoma telomerase RNA assembles Tetrahymena protein components into a telomerase RNP complex. However, the lower RNP complex detected with the Gc.TER1 probe ran as a slightly broader band than that visualized with the Tt.TER1 probe, suggesting that conformational differences exist between the RNP complexes containing the two different RNAs. In Western blotting of the RNP gels, antibody signals from the upper complex diminished with more stringent washing, and other experiments indicated that some of the nucleic acid hybridization signal from this complex came from genomic DNA present in the S100 extract (T. Ware and E.H.B., unpublished observations). Previous studies of purified telomerase in the ciliate Euplotes aediculatus have shown only a single RNP complex (20). It is possible that the upper complex may represent a form of the telomerase holoenzyme, with partially masked protein epitopes associated with genomic DNA.
Incorporation of Variant Telomeric DNA Repeats in Vivo.
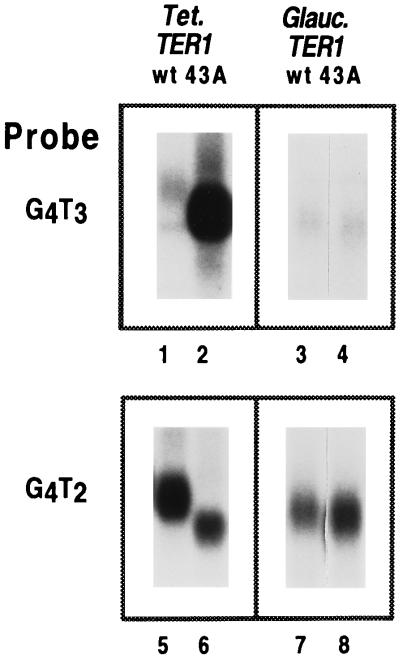
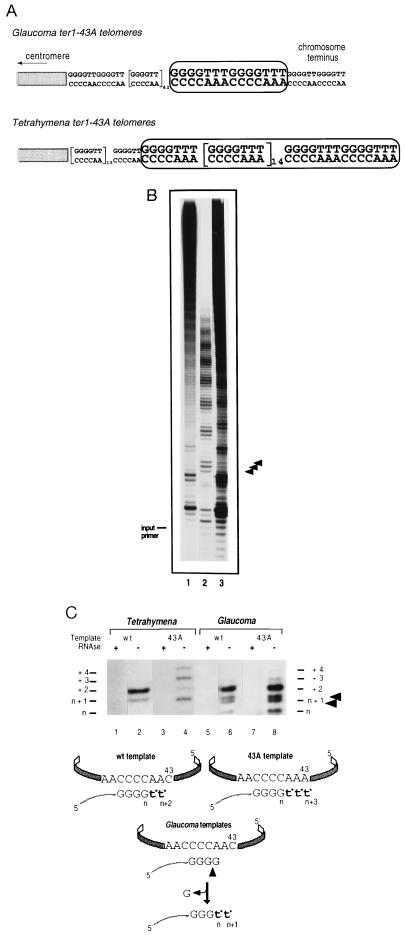
To address whether Gc.ter1–43A transformants produced enzymatically active telomerase, we first used Southern blotting analysis to detect G4T3 repeats in the macronuclear telomeres of transformant cells using a G4T3 repeat-specific probe (17) (Fig. 3). Compared with control Tt.ter1–43A transformants, Gc.ter1–43A transformants showed only low levels of G4T3 repeats (Fig. 3). To obtain an estimate of the relative numbers of telomeres containing G4T3 repeats, partial libraries of cloned telomeres were screened with G4T3- and G4T2-specific probes. In a Gc.ter1–43A telomere library, three cloned telomeres contained G4T3 repeats as well as G4T2 repeats, and 41 contained only G4T2 repeats. Of the cloned telomeres analyzed from a control Tt.ter1–43A library, five clones had G4T3 repeats, and 40 contained only G4T2 repeats. These results suggested that the Gc.ter1–43A and Tt.ter1–43A enzymes synthesized repeats onto comparable numbers of telomeres in vivo. However, DNA sequence analysis of two of the G4T3-containing clones from the Gc.ter1–43A library showed that the Gc.ter1–43A enzyme had added only two contiguous repeats in both clones (Fig. 4A). In contrast, in four cloned telomeres from the Tt.ter1–43A library, 16–17 consecutive G4T3 repeats were found in each clone.
Figure 3.
Southern blot analysis of genomic DNA from T. thermophila transformants containing Tetrahymena and Glaucoma TER1 and ter1–43A genes. PstI-cut transformant genomic DNAs were separated by 0.7% agarose gel electrophoresis and subjected to Southern blot analysis. Transformant macronuclear rDNA telomeres were analyzed using either a G4T3- (lanes 1–4) or a G4T2-specific (lanes 5–8) oligonucleotide probe (17). Lanes 5–8 are duplicate lanes to lanes 1–4. Only part of the Southern blot containing the rDNA telomeres is shown.
Figure 4.
Incorporation of G4T3 variant telomeric repeats in vivo and in vitro. (A) DNA sequences of representative G4T3-containing telomere clones isolated from Gc.ter1–43A and Tt.ter1–43A transformant telomere libraries. Boxes highlight occurrence of variant G4T3 repeats. (B) In vitro telomerase activity from Tt.TER1 (lane 1), Tt.ter1–43A (lane 2), and Gc.ter1–43A (lane 3) extracts in presence of labeled dGTP and unlabeled dTTP. Arrows indicate positions of G4T3 repeat products in Glaucoma ter1–43A extract amid the largely wild-type repeat pattern, consistent with only two rounds of 43A-templated elongation synthesis. Size of unlabeled input primer is indicated. (C) In vitro telomerase activity from transformant extracts in the presence of [α-32P] dTTP-only. Lanes 1, 3, 5, and 7: telomerase fractions were pretreated with RNase A. Lane 2, extension products from Tt.TER1; lane 4, Tt.ter1–43A extracts. Lengths of control DNA fragments are indicated. Schematic summaries represent products predicted from wild-type or 43A telomerase RNA template. The lower band in the n + 1 doublet (arrowed, lanes 6 and 8) likely represents cleavage of the 3′-terminal dG from primer d(T2G4)3 and addition of two radiolabeled dT* bases, producing a d(T2G4)2TTGGGT*T* product. This species is expected to migrate slightly faster than the d(T2G4)2TTGGGGT* product, generated by a single dT* residue addition onto the uncleaved primer.
Hybrid Glaucoma Telomerase Enzyme Functions Aberrantly in Vitro.
We directly assessed the enzymological properties of the Gc.TER1 telomerase in vitro. Telomerase activity, prepared from Gc.TER1 and Gc.ter1–43A transformants and from Tt.TER1 or Tt.ter1–43A transformant controls, was compared in complete telomerase reactions containing radiolabeled dGTP and unlabeled dTTP (G + T reaction) primed with the telomeric oligonucleotide d(T2G4)3. The Tt.TER1 and Tt.ter1–43A extracts produced the described (4) patterns of 6-base G4T2 or 7-base G4T3 repeats, respectively (Fig. 4B, lanes 1 and 2). In contrast, the Gc.ter1–43A transformant activity produced the largely wild-type 6-base repeat pattern of the endogenous Tetrahymena telomerase activity (Fig. 4B, lane 3). However, there were additional, clearly visible bands corresponding to up to two G4T3 repeats (arrowheads, Fig. 4B). This pattern differed from that of Tt.ter1–43A transformants, in which the bands corresponding to addition of three and four G4T3 repeats were stronger than those from addition of two repeats (Fig. 4B, lane 2). In control experiments, such longer G4T3 repeat products were clearly apparent when low ratios of telomerase activity from Tt.ter1–43A transformants were mixed with Tt.TER1 extracts and assayed (data not shown). Both Gc.ter1–43A and Tt.ter1–43A RNAs and RNPs were present in comparable amounts in the Gc.ter1–43A transformant cells. Hence, the hybrid Gc.ter1–43A RNP in Tetrahymena cells was not only enzymatically less active but also was less processive than its all-Tetrahymena counterpart enzyme.
The Glaucoma RNA-substituted telomerase was assayed with radiolabeled dTTP alone (T*-only reaction), using a d(T2G4)3 oligonucleotide primer. In control reactions, the Tt.TER1 (wild-type) telomerase added two dT* residues (n + 2 product, where the primer is n nucleotides long) to the 3′ end of this dG4-ending primer (Fig. 4C, schematic and lane 2), and the Tt.ter1–43A enzyme added the predicted three dT* residues (n + 3 product) as has been shown (4) (Fig. 4C, schematic and lane 4). As described (4), the Tt.ter1–43A telomerase activity “stuttered,” incorporating an extra dT* nucleotide in addition to the predicted three-templated dT* bases (Fig. 4C, lane 4). The Gc.ter1–43A activity also synthesized the predicted n + 3, labeled product, plus the analogous n + 4 band (Fig. 4C, lane 8).
In marked contrast to the Tt.TER1 or Tt.ter1–43A enzyme activities, both the Gc.TER1 and Gc.ter1–43A activities produced a labeled, primer-sized product (n) plus a labeled doublet at the n + 1 position (Fig. 4C, lanes 6 and 8, arrowheads). These products were all sensitive to RNase pretreatment. The primer-sized (n), labeled band must contain at least one labeled dT* residue and can be explained by the presence of a telomerase-based cleavage activity, induced by the presence of Glaucoma RNA in the telomerase RNP, removing one (or more) dG residues from the 3′ end of the DNA primer and adding one (or more) labeled dT* residues. Because only dTTP was present, the labeled n + 1 doublet represents two products of the same length but different base composition. We deduced that the lower band was generated by 3′ cleavage and two dT* additions, and the upper band (which comigrated with the n + 1 product in Fig. 4C, lane 4; data not shown) was generated from a dT* addition directly to the uncleaved primer (Fig. 4C, lanes 6 and 8, arrows and schematic). Such cleavage and 3′ replacement reactions have been reported for wild-type Tetrahymena and Euplotes telomerases with certain primers but have not been reported for (T2G4)3 or comparable primers (21, 22).
DISCUSSION
Role of Telomerase RNA Domains in Enzymatic Function in Vivo.
In this first reported cross-species telomerase RNA swap, remarkably, a telomerase RNA that was ≈50% divergent outside the template region assembled and functioned in vivo. Nevertheless, this new hybrid enzyme had lower activity and polymerized repeats less processively. It also displayed an aberrant cleavage activity. We propose that the compromised polymerization and miscleavage resulted from altered RNA–protein and/or protein–protein interactions in the hybrid telomerase. Specifically, these appear to place the cleavage active site in an altered spatial relationship to the template/primer. The telomerase cleavage activity was reminiscent of nascent transcript cleavages stimulated by transcription elongation factors in eukaryotes and prokaryotes (23–26), proposed to relieve “dead-end” transcription complexes (27, 28). Thus, it is possible that the aberrant cleavage and lowered processivity of the hybrid telomerase may be mechanistically related.
We had suggested a domain model of the telomerase RNA based on function (15), with the template region associated with enzymatic function and other regions of the RNA that might be involved more directly with binding telomerase protein components. This work reveals a new class of telomerase RNA mutants, demonstrating that telomerase RNA domains other than the template domain affect crucial molecular events taking place at the polymerization active site. Thus, our present study indicates that these “domain-associated” functions are not mutually exclusive and in fact likely influence each other.
This work raises the intriguing possibility that telomerase activity can be controlled or regulated by changing the molecular interactions involving its nontemplate RNA domains. At present, it is not known if specific telomerase RNA structural elements affect specific telomerase enzymatic functions. Pleij and colleagues (29) have proposed, for example, that the phylogenetically conserved helix III element (3′ to the template region; Fig. 1A) adopts a pseudoknot conformation. We had postulated (15) that the helix III region in the naked telomerase RNA in vitro was conformationally flexible, likely undergoing transitions between hairpin and pseudoknot states, while in the telomerase RNP the pseudoknot form might be stabilized by protein interactions. It also has been speculated that this helix-to-pseudoknot RNA structural transition might affect polymerization events at the template (15, 16). Thus, elucidating the RNA determinants of telomerase action may identify RNP domains that interact with naturally occurring telomerase control factors and point to new drug targets in the RNP for control of telomerase activity in vivo.
Acknowledgments
We thank Karen Kirk, Jagoree Roy, H. E. Wang, and Tracy Ware for critical reading of the manuscript. We are indebted to Alan Frankel for providing helpful comments on the manuscript. This work was funded by a Lucille P. Markey Charitable Trust Visiting Postdoctoral Fellowship and American Cancer Society (California Division) Senior Fellowship (A.B.). Laboratory support also was provided in part by the Lucille P. Markey Charitable Trust and by National Institutes of Health Grant GM 26259 (E.H.B.).
ABBREVIATION
- RNP
ribonucleoprotein
References
- 1.Telomeres (1995) eds. Greider C. & Blackburn E. H. (Cold Spring Harbor Lab. Press, Plainview, NY).
- 2.Kirk, K. E., Harmon, B. P., Riechardt, I. K, Sedat, J. & Blackburn, E. H. (1997) Science, in press. [DOI] [PubMed]
- 3.Greider C W, Blackburn E H. Nature (London) 1989;337:331–337. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- 4.Gilley D, Lee M S, Blackburn E H. Genes Dev. 1995;9:2214–2226. doi: 10.1101/gad.9.18.2214. [DOI] [PubMed] [Google Scholar]
- 5.Gilley D, Blackburn E H. Mol Cell Biol. 1996;16:66–75. doi: 10.1128/mcb.16.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zahler A M, Prescott D M. Nucleic Acids Res. 1988;16:6953–6972. doi: 10.1093/nar/16.14.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shippen-Lentz, D. E. & Blackburn, E. H. Science 247, 546–552. [DOI] [PubMed]
- 8.Singer M S, Gottschling D E. Science. 1994;266:404. doi: 10.1126/science.7545955. [DOI] [PubMed] [Google Scholar]
- 9.McEachern M J, Blackburn E H. Nature (London) 1995;376:403–409. doi: 10.1038/376403a0. [DOI] [PubMed] [Google Scholar]
- 10.Feng J, Funk W D, Wang S-S, Weinrich S L, Avilion A A, Chiu C-P, Adams R R, Chang E, Allsopp R C, Yu J, Le S, West M D, Harley C B, Andrews W H, Greider C W, Villeponteau B. Science. 1995;269:1236–1241. doi: 10.1126/science.7544491. [DOI] [PubMed] [Google Scholar]
- 11.Blasco M A, Funk W, Villeponteau B, Greider C W. Science. 1995;269:1267–1270. doi: 10.1126/science.7544492. [DOI] [PubMed] [Google Scholar]
- 12.Romero D P, Blackburn E H. Cell. 1991;67:343–353. doi: 10.1016/0092-8674(91)90186-3. [DOI] [PubMed] [Google Scholar]
- 13.Lingner J, Hendrick L L, Cech T R. Genes Dev. 1994;8:1984–1998. doi: 10.1101/gad.8.16.1984. [DOI] [PubMed] [Google Scholar]
- 14.McCormick-Graham M, Romero D P. Nucleic Acids Res. 1995;23:1091–1097. doi: 10.1093/nar/23.7.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhattacharyya A, Blackburn E H. EMBO J. 1994;13:5721–5731. doi: 10.1002/j.1460-2075.1994.tb06910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaug A J, Cech T R. RNA. 1995;1:363–374. [PMC free article] [PubMed] [Google Scholar]
- 17.Kirk K E, Blackburn E H. Genes Dev. 1995;9:59–71. doi: 10.1101/gad.9.1.59. [DOI] [PubMed] [Google Scholar]
- 18.Yu G-L, Bradley J D, Attardi L D, Blackburn E H. Nature (London) 1990;344:126–132. doi: 10.1038/344126a0. [DOI] [PubMed] [Google Scholar]
- 19.Collins K, Kobayashi R, Greider C W. Cell. 1995;81:677–686. doi: 10.1016/0092-8674(95)90529-4. [DOI] [PubMed] [Google Scholar]
- 20.Lingner J, Cech T R. Proc Natl Acad Sci USA. 1996;93:10712–10717. doi: 10.1073/pnas.93.20.10712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins K, Greider C W. Genes Dev. 1993;7:1364–1376. doi: 10.1101/gad.7.7b.1364. [DOI] [PubMed] [Google Scholar]
- 22.Melek M, Greene E C, Shippen D E. Mol Cell Biol. 1996;16:3437–3445. doi: 10.1128/mcb.16.7.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng G H, Lee D N, Chan C L, Landick R. J Biol Chem. 1994;269:22282–22294. [PubMed] [Google Scholar]
- 24.Stebbins C E, Borukhov S, Orlova M, Polyakov A, Goldfarb A, Darst S A. Nature (London) 1995;373:636–640. doi: 10.1038/373636a0. [DOI] [PubMed] [Google Scholar]
- 25.Reines D, Ghanouni P, Li Q Q, Mote J., Jr J Biol Chem. 1992;267:15516–15522. [PMC free article] [PubMed] [Google Scholar]
- 26.Jeon C, Yoon H, Agarwal K. Proc Natl Acad Sci USA. 1994;91:9106–9110. doi: 10.1073/pnas.91.19.9106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erie D A, Hajiseyedjavadi O, Young M C, von Hippel P H. Science. 1993;262:867–873. doi: 10.1126/science.8235608. [DOI] [PubMed] [Google Scholar]
- 28.Lee D N, Feng G, Landick R. J Biol Chem. 1994;269:22295–22303. [PubMed] [Google Scholar]
- 29.ten Dam E, van Belkum A, Pleij C W A. Nucleic Acids Res. 1991;19:6951. doi: 10.1093/nar/19.24.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]