Abstract
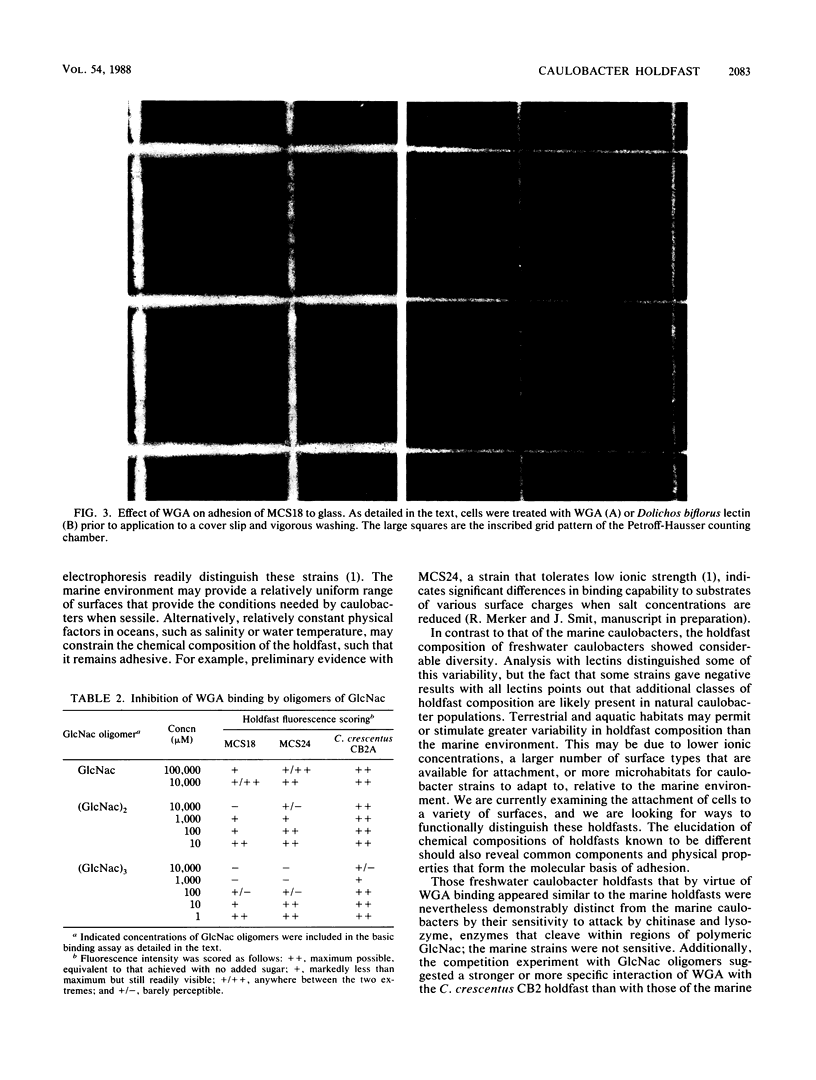
Caulobacters are prosthecate (stalked) bacteria that elaborate an attachment organelle called a holdfast at the tip of the cellular stalk. We examined the binding of lectins to the holdfasts of 16 marine Caulobacter strains and 10 freshwater species or strains by using a panel of fluorescein-conjugated lectins and fluorescence microscopy. The holdfasts of all the marine isolates bound to only wheat germ agglutinin (WGA) and other lectins that bind N-acetylglucosamine (GlcNac) residues. The freshwater caulobacters showed more variability in holdfast composition. Some bound only to WGA and comparable lectins as the marine strains did. Others bound additional or other lectins, and some did not bind to the lectins tested. The binding of WGA appeared to involve the regions of the holdfast involved with adhesion; a holdfast bound to WGA was significantly less adhesive to glass. Competition experiments with WGA-binding holdfasts and oligomers of GlcNac demonstrated that trimers of GlcNac (the preferred substrate for WGA binding) were more effective than dimers or monomers in preventing WGA binding to holdfasts, suggesting that stretches of contiguous GlcNac residues occur in the WGA-binding holdfasts. In addition, differences between freshwater and marine holdfasts in the strength of WGA binding were noted. The effect of a number of proteolytic and glycolytic enzymes on holdfast integrity was examined; the proteases had no effect for all caulobacters. None of the glycolytic enzymes had an effect on marine caulobacter holdfasts, but chitinase and lysozyme (both attack oligomers of GlcNac) disrupted the holdfasts of those freshwater caulobacters that bound WGA. Despite some similarity to chitin, holdfasts did not bind Calcofluor and no measurable effects on holdfast production were detectable after cell growth in the presence of diflubenzuron or polyoxin D, inhibitors of chitin synthesis in other systems. Finally, the holdfasts of all caulobacters bound to colloidal gold particles, without regard to the coating used to stabilize the gold particles. This binding was stronger or more specific than WGA binding; treatment with colloidal gold particles prevented WGA binding, but the reverse was not the case.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anast Nick, Smit John. Isolation and Characterization of Marine Caulobacters and Assessment of Their Potential for Genetic Experimentation. Appl Environ Microbiol. 1988 Mar;54(3):809–817. doi: 10.1128/aem.54.3.809-817.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann P., Baumann L., Mandel M. Taxonomy of marine bacteria: the genus Beneckea. J Bacteriol. 1971 Jul;107(1):268–294. doi: 10.1128/jb.107.1.268-294.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belas R., Simon M., Silverman M. Regulation of lateral flagella gene transcription in Vibrio parahaemolyticus. J Bacteriol. 1986 Jul;167(1):210–218. doi: 10.1128/jb.167.1.210-218.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Cheng K. J., Geesey G. G., Ladd T. I., Nickel J. C., Dasgupta M., Marrie T. J. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987;41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Irvin R. T., Cheng K. J. The bacterial glycocalyx in nature and disease. Annu Rev Microbiol. 1981;35:299–324. doi: 10.1146/annurev.mi.35.100181.001503. [DOI] [PubMed] [Google Scholar]
- Debray H., Decout D., Strecker G., Spik G., Montreuil J. Specificity of twelve lectins towards oligosaccharides and glycopeptides related to N-glycosylproteins. Eur J Biochem. 1981 Jun;117(1):41–55. doi: 10.1111/j.1432-1033.1981.tb06300.x. [DOI] [PubMed] [Google Scholar]
- Endo A., Kakiki K., Misato T. Mechanism of action of the antifugal agent polyoxin D. J Bacteriol. 1970 Oct;104(1):189–196. doi: 10.1128/jb.104.1.189-196.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazio S. A., Uhlinger D. J., Parker J. H., White D. C. Estimations of uronic acids as quantitative measures of extracellular and cell wall polysaccharide polymers from environmental samples. Appl Environ Microbiol. 1982 May;43(5):1151–1159. doi: 10.1128/aem.43.5.1151-1159.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giloh H., Sedat J. W. Fluorescence microscopy: reduced photobleaching of rhodamine and fluorescein protein conjugates by n-propyl gallate. Science. 1982 Sep 24;217(4566):1252–1255. doi: 10.1126/science.7112126. [DOI] [PubMed] [Google Scholar]
- Gordon A. S., Millero F. J. Electrolyte effects on attachment of an estuarine bacterium. Appl Environ Microbiol. 1984 Mar;47(3):495–499. doi: 10.1128/aem.47.3.495-499.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gristina A. G. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science. 1987 Sep 25;237(4822):1588–1595. doi: 10.1126/science.3629258. [DOI] [PubMed] [Google Scholar]
- Ker R. F. Investigation of locust cuticle using the insecticide diflubenzuron. J Insect Physiol. 1977;23(1):39–48. doi: 10.1016/0022-1910(77)90107-x. [DOI] [PubMed] [Google Scholar]
- Leighton T., Marks E., Leighton F. Pesticides: insecticides and fungicides are chitin synthesis inhibitors. Science. 1981 Aug 21;213(4510):905–907. doi: 10.1126/science.213.4510.905. [DOI] [PubMed] [Google Scholar]
- Maeda H., Ishida N. Specificity of binding of hexopyranosyl polysaccharides with fluorescent brightener. J Biochem. 1967 Aug;62(2):276–278. doi: 10.1093/oxfordjournals.jbchem.a128660. [DOI] [PubMed] [Google Scholar]
- Nachbar M. S., Oppenheim J. D., Thomas J. O. Lectins in the U.S. Diet. Isolation and characterization of a lectin from the tomato (Lycopersicon esculentum). J Biol Chem. 1980 Mar 10;255(5):2056–2061. [PubMed] [Google Scholar]
- POINDEXTER J. S. BIOLOGICAL PROPERTIES AND CLASSIFICATION OF THE CAULOBACTER GROUP. Bacteriol Rev. 1964 Sep;28:231–295. doi: 10.1128/br.28.3.231-295.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poindexter J. S. The caulobacters: ubiquitous unusual bacteria. Microbiol Rev. 1981 Mar;45(1):123–179. doi: 10.1128/mr.45.1.123-179.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit J., Grano D. A., Glaeser R. M., Agabian N. Periodic surface array in Caulobacter crescentus: fine structure and chemical analysis. J Bacteriol. 1981 Jun;146(3):1135–1150. doi: 10.1128/jb.146.3.1135-1150.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smucker R. A., Simon S. L. Some Effects of Diflubenzuron on Growth and Sporogenesis in Streptomyces spp. Appl Environ Microbiol. 1986 Jan;51(1):25–31. doi: 10.1128/aem.51.1.25-31.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley J. T., Jordan T. L. Crossbands of Caulobacter crescentus stalks serve as indicators of cell age. Nature. 1973 Nov 16;246(5429):155–156. doi: 10.1038/246155a0. [DOI] [PubMed] [Google Scholar]
- Sutherland I. W. Microbial exopolysaccharides -- their role in microbial adhesion in aqueous systems. Crit Rev Microbiol. 1983;10(2):173–201. doi: 10.3109/10408418209113562. [DOI] [PubMed] [Google Scholar]
- Waxdal M. J. Pokeweed mitogens. Methods Enzymol. 1978;50:354–361. doi: 10.1016/0076-6879(78)50042-6. [DOI] [PubMed] [Google Scholar]
- Zobell C. E. The Effect of Solid Surfaces upon Bacterial Activity. J Bacteriol. 1943 Jul;46(1):39–56. doi: 10.1128/jb.46.1.39-56.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]