Abstract
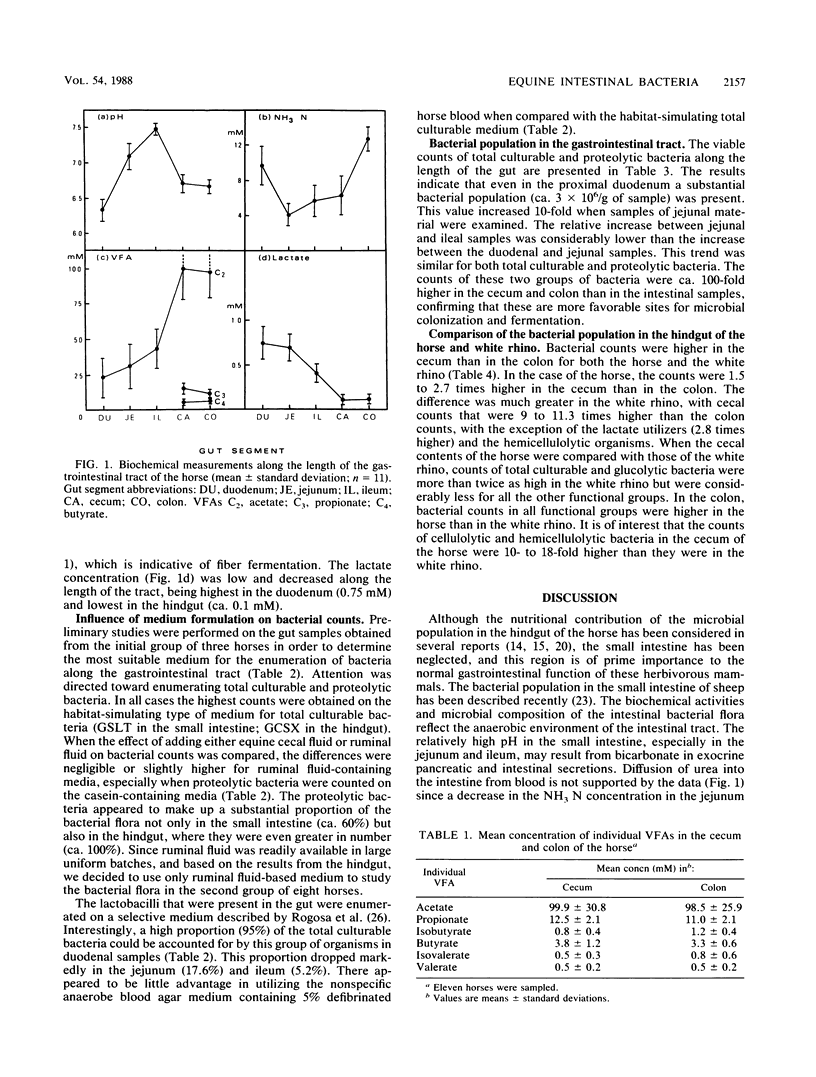
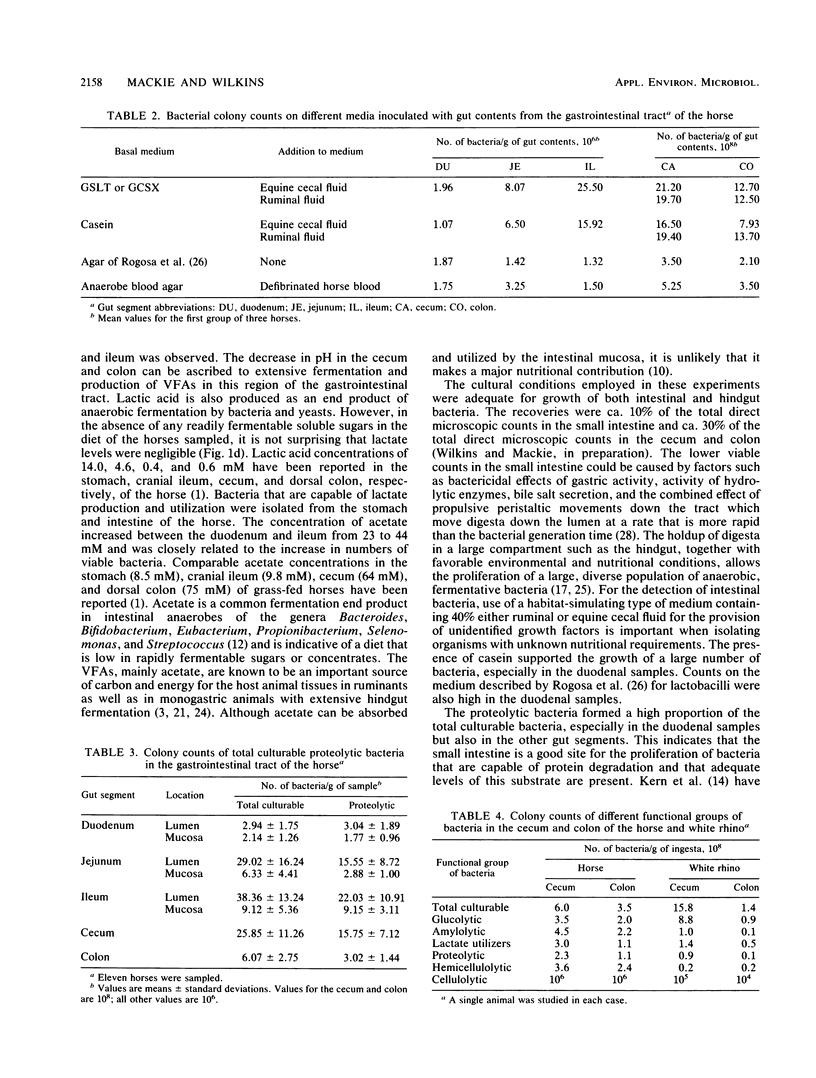
Samples from the duodenum, jejunum, and ileum, as well as from the cecum and colon, were obtained from 11 mature grass-fed horses. Viable counts of total culturable and proteolytic bacteria were made on habitat-simulating media containing 40% clarified ruminal fluid. The mean pHs in the duodenum, jejunum, and ileum were 6.32, 7.10, and 7.47, respectively; the mean pH decreased to 6.7 in the hindgut. The acetate concentration increased along the length of the small intestine and was the only volatile fatty acid present in this gut segment. Molar proportions of acetate, propionate, and butyrate in the hindgut were 85:10:3. Differences in bacterial counts on habitat-simulating media containing equine cecal fluid or clarified ruminal fluid were negligible. Bacterial counts showed a substantial population in the duodenum (ca. 2.9 x 10(6) per g [wet weight] of sample), and this increased to 29.0 x 10(6) in the jejunum and 38.4 x 10(6) in the ileum. Proteolytic bacteria formed a high proportion of the total culturable bacteria, especially in duodenal samples. Counts of proteolytic bacteria per gram (wet weight) of sample were 3.0 x 10(6), 15.6 x 10(6), and 22.0 x 10(6) in the duodenum, jejunum, and ileum, respectively. There was a close relationship between lumenal and mucosal bacterial counts, although actual values were lower in mucosal samples. The mucosal bacterial population in the duodenum was high relative to the lumenal population. Although the comparison of bacterial populations in the hindgut of the horse and white rhino was limited to a single animal, the results were of interest. Counts were higher in the cecum than in the colon for both the horse and the white rhino.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER F., DAVIES M. E. Production and fermentation of lactate by bacteria in the alimentary canal of the horse and pig. J Comp Pathol. 1963 Jan;73:1–8. doi: 10.1016/s0368-1742(63)80001-6. [DOI] [PubMed] [Google Scholar]
- Argenzio R. A., Stevens C. E. The large bowel--a supplementary rumen? Proc Nutr Soc. 1984 Jan;43(1):13–23. doi: 10.1079/pns19840022. [DOI] [PubMed] [Google Scholar]
- CHANEY A. L., MARBACH E. P. Modified reagents for determination of urea and ammonia. Clin Chem. 1962 Apr;8:130–132. [PubMed] [Google Scholar]
- Czerkawski J. W. The use of pivalic acid as a reference substance in measurements of production of volatile fatty acids by rumen micro-organisms in vitro. Br J Nutr. 1976 Sep;36(2):311–315. doi: 10.1079/bjn19760085. [DOI] [PubMed] [Google Scholar]
- Henning P. A. Examination of methods for enumerating hemicellulose-utilizing bacteria in the rumen. Appl Environ Microbiol. 1979 Jul;38(1):13–17. doi: 10.1128/aem.38.1.13-17.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern D. L., Slyter L. L., Leffel E. C., Weaver J. M., Oltjen R. R. Ponies vs. steers: microbial and chemical characteristics of intestinal ingesta. J Anim Sci. 1974 Mar;38(3):559–564. doi: 10.2527/jas1974.383559x. [DOI] [PubMed] [Google Scholar]
- Kern D. L., Slyter L. L., Weaver J. M., Leffel E. C., Samuelson G. Pony cecum vs. steer rumen: the effect of oats and hay on the microbial ecosystem. J Anim Sci. 1973 Aug;37(2):463–469. doi: 10.2527/jas1973.372463x. [DOI] [PubMed] [Google Scholar]
- Maczulak A. E., Dawson K. A., Baker J. P. Nitrogen utilization in bacterial isolates from the equine cecum. Appl Environ Microbiol. 1985 Dec;50(6):1439–1443. doi: 10.1128/aem.50.6.1439-1443.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J. H., Mackie R. I. Microbiological evaluation of the intraruminal in sacculus digestion technique. Appl Environ Microbiol. 1986 Mar;51(3):622–629. doi: 10.1128/aem.51.3.622-629.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti J. M., Davis C. L., Hespell R. B., Leedle J. A. Enumeration and presumptive identification of bacteria from the small intestine of sheep. J Dairy Sci. 1984 Jun;67(6):1227–1235. doi: 10.3168/jds.S0022-0302(84)81428-9. [DOI] [PubMed] [Google Scholar]
- ROGOSA M., MITCHELL J. A., WISEMAN R. F. A selective medium for the isolation and enumeration of oral and fecal lactobacilli. J Bacteriol. 1951 Jul;62(1):132–133. doi: 10.1128/jb.62.1.132-133.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C. Microorganisms associated with epithelial surfaces and stability of the indigenous gastrointestinal microflora. Nahrung. 1987;31(5-6):383–395. doi: 10.1002/food.19870310511. [DOI] [PubMed] [Google Scholar]
- Van Hoven W., Gilchrist F. M., Hamilton-Attwell V. L. Intestinal ciliated protozoa of African rhinoceros: two new genera and five new species from the white rhino (Ceratotherium simum Burchell, 1817). J Protozool. 1987 Aug;34(3):338–342. doi: 10.1111/j.1550-7408.1987.tb03186.x. [DOI] [PubMed] [Google Scholar]
- White N. What's next in equine colic research? Equine Vet J. 1986 Nov;18(6):429–431. doi: 10.1111/j.2042-3306.1986.tb03678.x. [DOI] [PubMed] [Google Scholar]



